Article Text
Abstract
Objectives: To estimate the effect of intravenous and nebulised magnesium sulphate upon hospital admissions and pulmonary function in adults and children with acute asthma.
Methods: We undertook a systematic review and meta-analysis of randomised and quasi-randomised trials of intravenous or nebulised magnesium sulphate in acute asthma. Trials were identified by searches of the electronic literature, relevant journal websites and conference proceedings, and contact with authors and experts. Data were pooled using random effects meta-analysis of the relative risk (RR) of hospital admission and the standardised mean difference (SMD) in pulmonary function.
Results: 24 studies (15 intravenous, 9 nebulised) incorporating 1669 patients were included. Intravenous treatment was associated in adults with weak evidence of an effect upon respiratory function (SMD 0.25, 95% confidence interval (CI) −0.01 to 0.51; p = 0.05), but no significant effect upon hospital admission (RR 0.87, 95% CI 0.70 to 1.08; p = 0.22), and in children with a significant effect upon respiratory function (SMD 1.94, 95% CI 0.80 to 3.08; p<0.001) and hospital admission (RR 0.70, 95% CI 0.54 to 0.90; p = 0.005). Nebulised treatment was associated in adults with weak evidence of an effect upon respiratory function (SMD 0.17, 95% CI −0.02 to 0.36; p = 0.09), and hospital admission (RR 0.68, 95% CI 0.46 to 1.02; p = 0.06), and in children with no significant effect upon respiratory function (SMD −0.26, 95% CI –1.49 to 0.98; p = 0.69) or hospital admission (RR 2.0, 95% CI 0.19 to 20.93; p = 0.56).
Conclusion: Intravenous magnesium sulphate appears to be an effective treatment in children. Further trials are needed of intravenous and nebulised magnesium sulphate in adults and nebulised magnesium sulphate in children.
Statistics from Altmetric.com
Asthma affects 5.2 million people in the UK, including 1.1 million children,1 and is responsible for around 60 000 hospital admissions per year.2 Guidelines from the British Thoracic Society (BTS) and Scottish Intercollegiate Guidelines Network (SIGN) advise a stepwise approach to the management of exacerbations.3 Initially all patients should receive oxygen; nebulised β2-agonists; nebulised anticholinergic agent and corticosteroids. However, bronchodilators act within minutes whereas corticosteroids require hours. This discrepancy suggests a role for magnesium as an alternative treatment option in patients resistant to standard therapy. Magnesium’s pharmacological action is based upon its ability to inhibit the release of calcium from vesicles in the sarcoplasmic reticulum, resulting in bronchial smooth muscle relaxation.4
Magnesium has been evaluated in both the intravenous and nebulised dosage form. The aerosolised route offers the advantage of a quick onset of action and lower incidence of side effects. Its disadvantages include a lower percentage of drug being delivered to the site of action and the patient requiring some respiratory effort to maximise its effectiveness. The intravenous route provides direct access to the venous system, allowing the delivery of high drug concentrations. The disadvantages include a cannula being sited and the drug being administered over 20 min.
Four meta-analyses have compared intravenous magnesium sulphate to placebo.5–8 Rowe et al5 identified five adult and two paediatric trials and concluded that magnesium sulphate therapy did not significantly improve peak expiratory flow rate or reduce admission to hospital, but subgroup analysis suggested that in trials of severe asthma, magnesium sulphate treatment was effective. Alter et al6 identified seven adult and two paediatric trials and found that magnesium sulphate was associated with a significant improvement in spirometric airway function by 16% of a standard deviation, but concluded that the clinical significance of this effect was uncertain. Rodrigo et al7 identified five adult trials and found no significant effect from magnesium sulphate upon pulmonary function or hospital admissions. Cheuk et al8 undertook a meta-analysis of five trials in children and concluded that intravenous magnesium sulphate was effective in reducing hospital admissions, and improving pulmonary function tests and clinical symptoms. Two reviews have compared nebulised magnesium sulphate to placebo.9 10 Both included six trials and concluded that current evidence could not clearly determine the role of nebulised magnesium sulphate in acute asthma.
The most recent (2007) BTS/SIGN guidelines state that a single dose of intravenous magnesium sulphate has been shown to be safe and effective in adults, and should be considered in adults with life threatening features or acute severe asthma that has not responded to inhaled bronchodilator treatment. The guidelines for children are more equivocal, suggesting that intravenous magnesium sulphate is safe but its place in management is not yet established. Nebulised magnesium sulphate is not discussed in either adults or children.
The evidence base for intravenous and nebulised magnesium sulphate has increased since these meta-analyses were published, with the recent publication of additional randomised trials. It is also apparent that magnesium sulphate may have a different role in adults and children. We therefore aimed to undertake a systematic review and meta-analysis of both intravenous and nebulised magnesium sulphate to determine their role in adults and children with acute asthma. Our specific objectives were to estimate the effect of each treatment upon pulmonary function and hospital admission.
METHODS
We planned to identify all randomised or quasi-randomised trials of intravenous or nebulised magnesium sulphate in adults or children with acute asthma that reported a measure of pulmonary function or hospital admission as an outcome.
The search terms “asthma” OR “wheeze” AND “magnes” were used to search the following databases: Cochrane Airways Review Group asthma register; Cochrane Clinical Trials Registry; Medline (1966-present); Medline in process (1966–present); EMBASE (1988–present); CINAHL (1982–present); AMED (1985–present); Research Registers of ongoing trials (MetaRegister of Current Controlled Trials (controlled-trials.com); National Research Register (NRR) and Centerwatch.com); Conference Papers Index; Web of Science; Dissertation Abstracts and the World Wide Web using the Google search engine.
We searched the websites of the following relevant journals: Emergency Medicine Journal; Academic Emergency Medicine; Thorax; Chest; European Respiratory Journal; Internet Scientific Journals/Journal Medical Internet Research (Emergency Medicine; Asthma, Allergy, Immunology; Pulmonary Medicine); Journal of Allergy and Clinical Immunology; Lancet; European Journal of Emergency Medicine; Annals of Emergency Medicine; American Journal Emergency Medicine; American Journal Respiratory and Critical Care Medicine; Journal of the American Medical Association; Journal of Asthma; British Medical Journal; Achives of Internal Medicine; Journal of Emergency Medicine. We also searched relevant conference proceedings for the previous 5 years for relevant trials: Society for Academic Emergency Medicine Annual Conference; Annual Thoracic Society International Conference; Annual Congress of European Respiratory Society; American College of Chest Physicians.
The reference lists of all articles selected were reviewed for relevant studies. The primary authors of included studies were contacted (where possible, determined by availability of an email address) for information on additional trials, both published and unpublished. Finally, clinicians, collaborators, colleagues and trialists were contacted to identify additional potentially relevant studies.
A single reviewer (SM) scanned titles and abstracts, searched journals, and contacted experts, and selected potentially relevant articles for review. When possible we retrieved the full version of selected articles. Two independent reviewers (SM and SG) then reviewed potentially relevant articles and selected definitely relevant articles for inclusion. Each reviewer also independently assessed the quality of each included study using the five point Jadad score. This scale is used to assess randomisation, double blinding and withdrawals/dropouts. All trials were scored using a scale of 1 to 5 (score of 5 being the highest).
The following data were extracted from each study: design (method of randomisation, withdrawals/dropouts, inclusion and exclusion criteria); participants (age, gender, severity of asthma); interventions (route of administration, dose, timing and duration of therapy, co-interventions); control (agents and doses used); outcomes (types of outcome measures and the timing of their measurements, hospital admission rates and side effects) and results. Unpublished data were requested from the primary author by email.
Data were analysed using RevMan statistical software (version 4.0). Since a variety of different pulmonary function measures were used in the trials, these measures were analysed as a standardised mean difference (SMD). Hospital admission was analysed as a relative risk. Both outcomes were pooled using a random effects model. Initially all studies were analysed together, then studies of adults and children were analysed separately.
RESULTS
The flow of identified studies through the selection process is shown in fig 1. The reviewers only disagreed on inclusion of one study.11 This study included patients with chronic obstructive pulmonary disease and was excluded after discussion. We thus identified 27 trials12–38 (23 published12–14 16–22 24–27 29–37 and four unpublished15 23 28 38) for inclusion. Three could not be included: one was only available in Chinese36 (nebulised magnesium, 75 patients), another did not report any of the outcome measures we intended to analyse37 (intravenous magnesium, 50 patients), and another was only available in abstract form and the authors could not be contacted38 (nebulised magnesium, 71 patients). We ultimately included 24 studies (15 intravenous and 9 nebulised) incorporating 1669 patients (1137 intravenous and 532 nebulised). Three of the studies were only available as abstracts15 23 28 but further details were obtained by contact with the authors. We also identified one ongoing trial of nebulised magnesium sulphate in children.39
Tables 1 and 2 show the characteristics of the studies: year of publication, country of origin, population characteristics, outcome measures and Jadad score. The studies showed heterogeneity in the age groups, severity of asthma and the exclusion criteria. Pulmonary function tests were used as the primary outcome measure by most studies. Hospital admissions were used as an outcome measure in 11/15 intravenous studies and 7/9 nebulised studies. The overall methodological quality of the studies included was generally high, with 16 out of 24 having a Jadad score of 4 or 5 (κ = 0.83).
Tables 3 and 4 show the interventions and co-interventions used in each study. Studies of intravenous magnesium used bolus doses ranging from 1.2–2 g (25–100 mg/kg for children). Only one study24 followed this with an infusion. Studies of nebulised magnesium showed substantial variation in the doses and number of nebulisations used. A placebo was used in all but two studies34 35 where magnesium alone was compared directly to a β-agonist (salbutamol).
Figure 2 shows the estimated effect of intravenous magnesium sulphate upon respiratory function and fig 3 shows the estimated effect upon hospital admission. In adults, treatment is associated with weak evidence of an effect upon respiratory function (SMD 0.25, 95% confidence interval (CI) −0.01 to 0.51; p = 0.05), but no significant effect upon hospital admission (relative risk (RR) 0.87, 95% CI 0.70 to 1.08; p = 0.22). In children, treatment is associated with a significant effect upon respiratory function (SMD 1.94, 95% CI 0.80 to 3.08; p<0.001) and hospital admission (RR 0.70, 95% CI 0.54 to 0.90; p = 0.005).
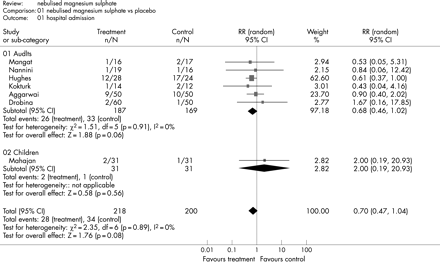
Figure 4 shows the estimated effect of nebulised magnesium sulphate upon respiratory function and fig 5 shows the estimated effect upon hospital admission. In adults, there is weak evidence of an effect upon respiratory function (SMD 0.17, 95% CI –0.02 to 0.36; p = 0.09) and hospital admission (RR 0.68, 95% CI 0.46 to 1.02; p = 0.06). In children, treatment is not associated with a significant effect upon respiratory function (SMD −0.26, 95% CI −1.49 to 0.98; p = 0.69) or hospital admission (RR 2.0, 95% CI 0.19 to 20.93; p = 0.56).
Two studies of nebulised magnesium sulphate34 35 compared magnesium sulphate to salbutamol instead of placebo. We re-analysed respiratory function data with these studies excluded. The results for adults were essentially unchanged (SMD 0.17, 95% CI –0.05 to 0.39; p = 0.13), whereas in children the one remaining study showed a significant effect from treatment (SMD 0.36, 95% CI –0.14 to 0.86; p = 0.05).
DISCUSSION
This is the most comprehensive review to date of the role of magnesium sulphate in acute asthma. Our analysis suggests that intravenous magnesium sulphate is an effective treatment in children, being associated with a significant improvement in respiratory function and a 30% decrease in hospital admissions. We found weak evidence that intravenous magnesium sulphate improves respiratory function in adults, but no evidence of a significant effect upon hospital admissions, although the data do not exclude a potential reduction in admissions of up to 30%. We found weak evidence that nebulised magnesium sulphate improves respiratory function and reduces hospital admissions in adults. Insufficient data exist to draw reliable conclusions regarding the role of nebulised magnesium sulphate in children. A trial is currently in progress to provide much-needed data on this last issue.39
Most previous meta-analyses have analysed adults and children together. Our analysis suggests that this may be inappropriate, particularly for intravenous magnesium sulphate, because there appears to be a clear difference in effectiveness between these two patient groups. It is not clear why effectiveness should differ between adults and children. Possible explanations are that children may have a greater element of reversibility to their acute asthma, or the use of weight adjusted dosing in children allows for a more appropriate dose of intravenous magnesium.
Our analysis has a number of potential limitations. Firstly, we may have failed to identify unpublished studies. We undertook a comprehensive literature search, including searches of conference abstracts, and identified three unpublished studies that were included in the review. Nevertheless we will not have identified studies that were neither presented nor published in any form. Secondly, we identified but were unable to include three potentially relevant studies because of limitations in their reporting. Thirdly, heterogeneity with respect to the exclusion criteria, treatment interventions and outcome measures may limit the appropriateness of pooling data. Of particular relevance and concern is the fact that the studies varied in whether patients with existing pulmonary pathology (such as chronic obstructive pulmonary disease) were excluded from the study. This is particularly significant as it is thought that patients with “pure” asthma are more likely to respond to magnesium treatment. Fourthly, most of the included studies were small and not powered to detect potentially important differences in hospital admission rates. Even after pooling these data we cannot exclude a potentially important effect from intravenous or nebulised magnesium sulphate in adults. Finally, we did not identify any studies that directly compared intravenous to nebulised magnesium sulphate.
Our analysis suggests that the revised (2007) BTS/SIGN guidelines are not entirely consistent with the current evidence. The guidelines suggest a clear role for intravenous magnesium sulphate in adults, but not children, and do not consider nebulised magnesium sulphate. In contrast, our analysis suggests that intravenous magnesium sulphate is an effective treatment for acute severe asthma in children, but has an uncertain role in adults. Nebulised and intravenous magnesium sulphate appear to be associated with similar estimates of effectiveness in adults, ranging from little or no effect to a substantial, worthwhile effect. Thus we can neither clearly state nor rule out a useful role for either nebulised of intravenous magnesium sulphate in adults.
The implications of our analysis are that intravenous magnesium sulphate should be standard treatment for children with acute severe asthma that has not responded to initial treatment, while the role of nebulised magnesium sulphate in children and the roles of both nebulised and intravenous magnesium sulphate in adults require further investigation. Given the low risk of serious side effects from magnesium sulphate it would seem reasonable to use intravenous magnesium sulphate in adults with life threatening features, in whom any potential benefit would justify the risks of treatment. Meanwhile, a large randomised trial is required to compare nebulised and intravenous magnesium sulphate to each other, and to placebo, in adults with acute severe asthma, to determine whether magnesium sulphate can improve symptoms and reduce hospital admissions. Further studies of nebulised magnesium sulphate in children are currently in progress.
Acknowledgments
The authors would like to thank Dr B Drobina, Professor A Greening and Dr S Bilaceroglu for providing the data from their study for inclusion into this review before publication. The authors would also like to thank the library staff based at the School of Health and Related Research, Sheffield, UK, for their assistance in undertaking the literature searches.
REFERENCES
Footnotes
Competing interests: SG is principal investigator for the 3Mg Trial, a multicentre trial of intravenous and nebulised magnesium sulphate in acute severe asthma.
- Abbreviations:
- BTS
- British Thoracic Society
- CI
- confidence interval
- RR
- relative risk
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMD
- standardised mean difference
Linked Articles
- Primary Survey