INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is considered a major nosocomial pathogen which causes diseases ranging in severity from skin and soft tissue infections to life-threatening conditions, and is associated with both a high clinical and a financial burden on healthcare systems [Reference Lindsay and Holden1–Reference Gould3]. Although MRSA has usually been considered within a hospital context, MRSA community-acquired infections have increasingly emerged as an important health problem [Reference Deleo4].The emergence and spread of MRSA is multifactorial in nature and is attributed to host factors, infection control practices and antibiotic use, and as such multiple intervention strategies are required to reduce MRSA infection rates [Reference Coia5–Reference Byrne and Wilcox8]. Antibiotic stewardship is considered a central component in multifaceted approaches to tackle the emergence and spread of antibiotic resistance [Reference Davey9, Reference Dellit10]; however, evidence on whether reducing antibiotic use will result in a parallel reduction in antibiotic resistance remains incomplete and further investigation is required.
In 2008, in response to controlling a hospital Clostridium difficile infection (CDI) outbreak, the Northern Health and Social Care Trust (NHSCT) in Northern Ireland implemented an enhanced antibiotic stewardship programme. This involved the restriction of high-risk antibiotic classes (i.e. second-generation cephalosporins, third-generation cephalosporins, fluoroquinolones, clindamycin) [Reference Aldeyab11, Reference Aldeyab12]. The restriction of high-risk antibiotics contributed to both a reduction in their use and a reduction in the incidence of CDI in the study site hospital [Reference Aldeyab12]. In addition, an educational activity to reduce the use of fluoroquinolones in the community setting was introduced. This policy was shown to be associated with a significant reduction in extended-spectrum β-lactamase-producing bacteria incidence rates in both hospital and community settings [Reference Aldeyab13].
The aim of the present investigation was to evaluate the impact of restricting high-risk antibiotics on MRSA incidence rates in a hospital setting. A secondary aim was to assess the impact of reducing fluoroquinolone use in the primary-care (community) setting on MRSA incidence in the community.
MATERIALS AND METHODS
Setting and study period
The NHSCT in N. Ireland consists of four hospitals, serving a population of about 420 000 people. The present study took place in one hospital (Causeway Hospital, 233 beds) since this hospital was not affected by the CDI outbreak which occurred in the NHSCT in 2008 [Reference Aldeyab11, Reference Aldeyab12]. Healthcare centres in primary care send their specimens to the NHSCT laboratory for assessment. The study was an ecological time-series analysis with a defined intervention period. The hospital intervention entailed restricting the use of high-risk antibiotics (second-generation cephalosporins, third-generation cephalosporins, fluoroquinolones, clindamycin), and the community intervention involved reducing the use of fluoroquinolones; both interventions commenced in January 2008. The study consisted of two components: (1) an evaluation of the impact of restricting the use of high-risk antibiotics in Causeway Hospital on MRSA incidence in that hospital; (2) an evaluation of the impact of reducing fluoroquinolone use in the NHSCT local community on MRSA incidence in that community. The present evaluation was conducted for the period January 2006 to June 2010, since data on some predictors (e.g. alcohol-base hand rub, age-adjusted comorbidity) were only available for that period. Details regarding the study site characteristics and implemented antibiotic stewardship are provided in a previous evaluation [Reference Aldeyab12].
Microbiology and pharmacy data
The number of new MRSA cases (hospital/community) was obtained from the clinical microbiology information system on a monthly basis. Hospital MRSA cases represent new cases that were identified during a patient's hospital stay (expressed per 100 bed-days). Community MRSA cases represent new cases identified in community samples sent to the Trust laboratory for analysis (per 1000 persons per day). Data allowing the distinction between MRSA colonized and infected patients were not available for Causeway Hospital for the whole study period. A sample was, however, considered for the period January 2010 to June 2010. MRSA isolates were processed according to routine microbiology procedures [Reference Aldeyab6]. Coagulase-positive isolates and their antimicrobial susceptibility were identified using the Vitek 2 system (bioMérieux, France). Monthly hospital antibiotic use was determined from the pharmacy information system and converted into defined daily doses (DDDs; ATC/DDD version 2010) [14]. Alcohol-based hand rub (litres) quantities issued to each ward each month were obtained from the pharmacy information system. Antibiotic use and alcohol gel data were normalized per 100 occupied bed-days. Age-adjusted comorbidity index (Charlson Index) was determined using data obtained from the Hospital Episode Statistics (HES) database [Reference Tobacman15]. Community antibiotic use was determined from the Business Services Organization (BSO) in N. Ireland and was expressed as DDDs/1000 persons per day.
Hospital antibiotic policy
The NHSCT implemented a revised antibiotic policy to minimize the use of high-risk antibiotics (second-generation cephalosporins, third-generation cephalosporins, fluoroquinolones, clindamycin; January 2008), and monitored the use of medium-risk antibiotics (i.e. amoxicillin-clavulanic acid and macrolides; September 2008). Clinical staff were encouraged to adhere to the hospital policy. Adherence to the implemented policy was continuously enforced and improved using audit and feedback and a pre-authorization requirement strategy [Reference Conlon16]. Approval for the use of restricted antibiotics which required authorization by a consultant and a subsequent assessment for appropriateness by the Antimicrobial Management Team (AMT), were used to observe the use of antimicrobials not included in the policy. The impact of this antibiotic policy on reducing high-risk antibiotic use has been reported in a previous investigation [Reference Aldeyab12].
NHSCT community intervention
In accordance with restricting fluoroquinolone use by patients served by the Trust, in order to manage a CDI outbreak in January 2008 [Reference Aldeyab11], a leaflet classifying fluoroquinolones as high-risk drugs was sent to all general practitioners (GPs) in the area. Reduced fluoroquinolone use was enforced and maintained via prescribing meetings with GPs, regular feedback (quarterly) on GPs' prescribing patterns, and training on appropriate antibiotic use. The impact of this antibiotic policy on reducing fluoroquinolone use in the community has been reported in a previous investigation [Reference Aldeyab13].
Statistical analysis
Segmented regression analysis of interrupted time-series was employed to evaluate the impact of restricting the use of high-risk antibiotics on MRSA incidence rates [Reference Wagner17]. This analysis allowed the estimation of changes between pre-intervention (January 2006 to December 2007) and intervention (January 2008 to June 2010) phases, while accounting for both sudden changes and the change trends of the outcome of interest. Monthly cases of MRSA were modelled as incidence rate. Analysis of the residuals of the fitted models showed that residuals were normally distributed (using the Jarque–Bera test), and there was no evidence of serial correlation (according to the Breusch–Godfrey test). In addition, analysis of the residual of the fitted models showed no evidence of heteroskedasticity (using the Breusch–Pagan–Godfrey test) with the exception of MRSA in community models; heteroskedasticity-adjusted standard errors were used for the latter series. Significance tests for parameter estimates were used to eliminate the unnecessary terms in the MRSA models in order to generate the most parsimonious model. A P value of <0·05 was considered to be statistically significant, and the most parsimonious MRSA model was selected. Analyses were performed using EViews 6 software (QMS, USA).
RESULTS
A total of 660 MRSA cases were identified in Causeway Hospital, while 1404 MRSA cases were identified in the local community over the study period (January 2006 to June 2010). Analysing a 6-month sample of data (January 2010 to June 2010) indicated that 23% and 97% of MRSA cases resulted from the clinical samples, for hospital and community, respectively, whereas the remaining cases resulted from screening patients. The average monthly MRSA incidence was 0·248/100 bed-days (range 0·103–0·415) and 0·002/1000 person-days (range 0·001–0·003), in the hospital and the local community, respectively. An increased trend in hospital alcohol-based hand rub use (P < 0·0001) was observed over the study period.
The introduction of the revised antibiotic policy intervention was not associated with a significant change in MRSA level (P = 0·5669) in Causeway Hospital; however, a significant change in trend was observed (P = 0·0057), with the MRSA incidence rate being reduced by 0·00561/100 bed-days per month. Analysis showed that variations in the incidence of hospital MRSA were affected by use of alcohol-based hand rub (coefficient = −0·045174, P = 0·0364, lag = 2 months; Table 1); 36% of the variation in the incidence of hospital MRSA was explained by the identified model (model a, Table 1). In the most parsimonious model, both trend change and alcohol-based hand rub use variables remained significant (model b, Table 1). Plots for monthly hospital MRSA incidence vs. use of high-risk antibiotic groups, and alcohol-based hand rub in the study site hospital are presented in Figure 1.

Fig. 1. Monthly hospital MRSA incidence vs. use of (a) high-risk antibiotic group (second-generation cephalosporins, third-generation cephalosporins, fluoroquinolones, clindamycin), and (b) alcohol-based hand rub, Causeway Hospital, January 2006 to June 2010. The vertical dashed line indicates the introduction of the intervention in January 2008. DDD, Defined daily dose.
Table 1. Parameter estimates from the full and most parsimonious segmented regression models assessing changes in MRSA incidence rates after the intervention, Causeway Hospital, January 2006 to June 2010

* Indicates the size and the direction of the effect; s.e., standard error.
† Lag time = 2 months; represents the delay necessary to observe the effect.
‡ AR, autoregressive term (order 2); representing past incidence density of MRSA.
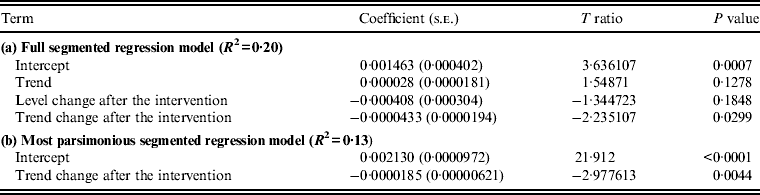
Analysis showed that the intervention relating to reducing fluoroquinolone use in the NHSCT local community was associated with a significant trend change in MRSA incidence in that area (P = 0·0299), with the MRSA incidence rate being reduced by 0·00004/1000 persons per day (model a, Table 2). There was no significant change in MRSA incidence in the community (P = 0·1848). In the most parsimonious model, trend change remained significant (model b, Table 2). Modelling the relationship between hospital MRSA series and community MRSA series showed insignificant correlation (coefficient = 0·000646, P = 0·5090). A plot for the monthly incidence of MRSA vs. use of fluoroquinolones in the NHSCT local community is presented in Figure 2.
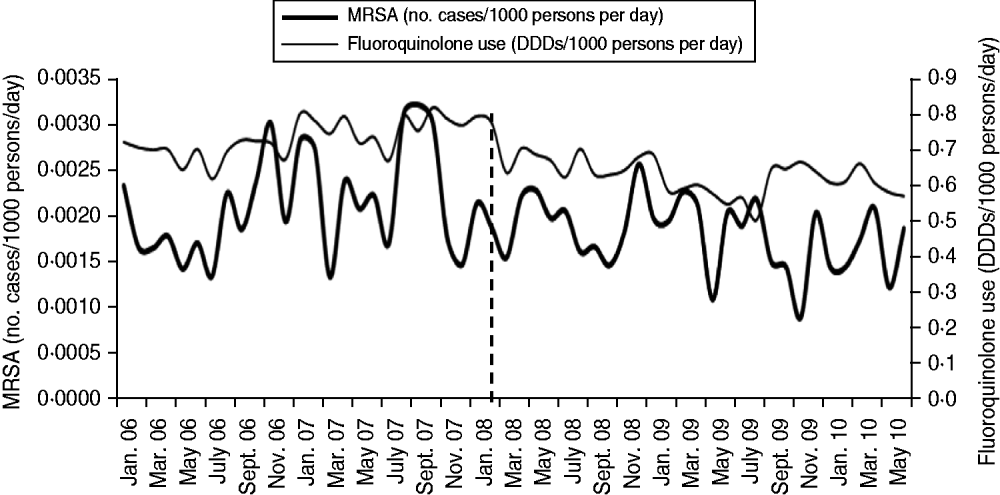
Fig. 2. Monthly MRSA incidence vs. use of fluoroquinolones, Northern Health and Social Care Trust local community, January 2006 to June 2010. The vertical dashed line indicates the introduction of the intervention in January 2008. DDD, Defined daily dose.
Table 2. Estimates from the segmented regression analysis assessing changes in the incidence of MRSA after the intervention, Northern Health and Social Care Trust local community, January 2006 to June 2010
DISCUSSION
Healthcare systems are currently facing several challenges requiring policy makers to prioritize their interventions and focus on the most practical and effective modifiable set of interventions to tackle healthcare-acquired infections. The findings of a previous investigation in Causeway Hospital (N. Ireland) showed that the restriction of high-risk antibiotics was the predominant factor in driving CDI incidence rate reduction [Reference Aldeyab12]. The present investigation aimed to evaluate the impact of reducing high-risk antibiotics on MRSA incidence rates within the same study site. Analysis of data showed that the incidence of hospital MRSA was significantly decreased following the restriction of high-risk antibiotics and adjusting for the use of alcohol gel-based hand rub. Such findings confirm our previous work on the importance of antibiotic use and infection control practices in the development of hospital-acquired MRSA, and provide further evidence in line with a cause–effect relationship between antibiotic use and resistance [Reference Aldeyab6]. The results are also consistent with evidence of the involvement of restricted antibiotics in increasing MRSA incidence rates in hospitals [Reference Aldeyab6, Reference Tacconelli18–Reference Lafaurie22].
Antibiotic consumption can create selection for resistance in a population while at the individual patient level antibiotics can modify the host's normal flora by eradicating the susceptible microorganisms, thus increasing the patient's probability of being colonized with a resistant organism [Reference Lipsitch and Samore23]. In addition, antibiotic use can increase the density of the resistant organisms carried by a patient which may enhance shedding of these organisms and increase the risk of acquisition by other exposed patients [Reference Lipsitch and Samore23]. Thus, the use of antibiotics not only has important implications for the spread and acquisition of MRSA in hospitals, but also in primary healthcare settings.
The restriction of certain antibiotic classes has been shown to be associated with positive clinical and microbiological outcomes in hospitals [Reference Davey9]. However, the implementation of antibiotic stewardship within primary healthcare settings is more difficult to achieve. In a previous study [Reference Aldeyab13], our group showed that the intervention relating to fluoroquinolone use in the NHSCT local community was associated with a significant reduction in their use. This reduction was achieved through continuous educational efforts as described earlier. Interestingly, the present study showed that this was also associated with a significant decrease in MRSA incidence trend in the community. The findings were consistent with the resistance patterns obtained from the Trust's microbiology department, which showed that MRSA in the community were resistant to ciprofloxacin in 95% of the cases examined.
Although the spread and acquisition of MRSA has been related to the use of different classes of antimicrobial therapy, a higher risk has been reported following therapy with fluoroquinolones in particular [Reference Tacconelli18]. Possible explanations could be related to the excretion of ciprofloxacin in sweat and across mucosal surfaces, thus promoting the spread of MRSA strains by eradication of susceptible microorganisms and increasing the expression of fibronectin-binding proteins on MRSA cell surfaces [Reference Lipsitch and Samore23, Reference Hoiby24]. A recently published systematic review and meta-analysis showed a consistent association, at the individual level, between receiving antibiotics and resistance in respiratory and urinary bacteria to those antibiotics [Reference Costelloe25]. Further, a controlled observational study on the effect of antibiotic prescribing in primary care on MRSA carriage, suggested that antibiotics prescribed within 12 months were associated with MRSA carriage [Reference Costelloe26].
Hand hygiene is considered as an essential strategy that reduces the risk of healthcare workers transmitting pathogens from one patient to another [Reference Coia5]. Despite the simplicity of this procedure, adherence remains low [Reference Sroka, Gastmeier and Meyer27]. The present study shows that the use of hospital alcohol-based hand rub was negatively correlated with the incidence of MRSA, i.e. an increase in the use of alcohol-based hand rub was associated with a decrease in MRSA rates, in accord with other published studies [Reference Aldeyab6, Reference Vernaz19, Reference Kaier20, Reference Sroka, Gastmeier and Meyer27, Reference Jarlier28]. Such findings highlight the importance of encouraging the use of alcohol-based hand rub, since there is much room for improvement in adherence, as one of the strategies in multifaceted approaches, to reduce MRSA rates in hospitals.
The study design has several strengths, including the use of the segmented regression of interrupted time-series analysis techniques which accounted for both sudden changes and the change trends of the outcome of interest. In addition, the model was improved by the inclusion of alcohol-based hand rub as a proxy measure for infection control practices. Data were collected as part of routine hospital practice independently from the study, therefore selection and information bias are unlikely. However, the study has some limitations: first, associations demonstrated by quasi-experimental studies at the population level may not correlate with associations at the individual patient level [Reference Harris, Lautenbach and Perencevich29, Reference Muller30]. Second, evaluation of the influence of restricting high-risk antibiotic use on MRSA incidence could have been improved if other possible factors (e.g. infection control activity including screening and isolation policies, and compliance with aseptic technique) had been available. However, the latter variables are likely to be involved in the variance which was not explained by the model presented. Third, in relation to the evaluation of MRSA incidence in Causeway Hospital, it was not possible to distinguish between MRSA cases that were acquired in the hospital or were positive on admission. Fourth, there is a lack of other possible factors that might explain the reduction of MRSA in the community. This latter area requires more investigation using data at the individual patient level.
In conclusion, the study showed that a reduction in high-risk antibiotic use and fluoroquinolone use contributed to a reduction in MRSA incidence rates in both hospital and community (primary care) settings.
DECLARATION OF INTEREST
None.
ACKNOWLEDGEMENTS
We thank Dominique L. Monnet (ECDC, Stockholm, Sweden) for expert advice and assistance on an earlier version of the manuscript.








