Abstract
Dexketoprofen trometamol is a water-soluble salt of the dextrorotatory enantiomer of the nonsteroidal anti-inflammatory drug (NSAID) ketoprofen. Racemic ketoprofen is used as an analgesic and an anti-inflammatory agent, and is one of the most potent in vitro inhibitors of prostaglandin synthesis. This effect is due to the (S)-(+)-enantiomer (dexketoprofen), while the (R)-(−)-enantiomer is devoid of such activity.
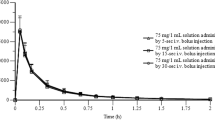
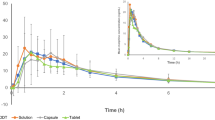
The racemic ketoprofen exhibits little stereoselectivity in its pharmacokinetics. Relative bioavailability of oral dexketoprofen (12.5 and 25mg, respectively) is similar to that of oral racemic ketoprofen (25 and 50mg, respectively), as measured in all cases by the area under the concentration-time curve values for (S)-(+)-ketoprofen. Dexketoprofen trometamol, given as a tablet, is rapidly absorbed, with a time to maximum plasma concentration (tmax) of between 0.25 and 0.75 hours, whereas the tmax for the (S)-(+)-enantiomer after the racemic drug, administered as tablets or capsules prepared with the free acid, is between 0.5 and 3 hours. The drug does not accumulate significantly when administered as 25mg of free acid 3 times daily. The profile of absorption is changed when dexketoprofen is ingested with food, reducing both the rate of absorption (tmax) and the maximal plasma concentration.
Dexketoprofen is strongly bound to plasma proteins, particularly albumin. The disposition of ketoprofen in synovial fluid does not appear to be stereoselective. Dexketoprofen trometamol is not involved in the accumulation of xenobiotics in fat tissues. It is eliminated following extensive biotransformation to inactive glucuroconjugated metabolites. No (R)-(−)-ketoprofen is found in the urine after administration of dexketoprofen, confirming the absence of bioinversion of the (S)-(+)-enantiomer in humans. Conjugates are excreted in urine, and virtually no drug is eliminated unchanged.
The analgesic efficacy of the oral pure (S)-(+)-enantiomer is roughly similar to that observed after double dosages of the racemic compound. At doses above 7mg, dexketoprofen was significantly superior to placebo in patients with moderate to severe pain. A dose-response relationship between 12.5 and 25mg could be seen in the time-effects curves, the superiority of the 25mg dose being more a result of an extended duration of action than of an increase in peak analgesic effect. A plateau in the analgesic activity of dexketoprofen trometamol at the 25mg dose is suggested. The time to onset of pain relief appeared to be shorter in patients treated with dexketoprofen trometamol. The drug was well tolerated.







Similar content being viewed by others
References
Veys EM. 20 years’ experience with ketoprofen. Scand J Rheumatol Suppl 1991; 90: 1–44
Cooper SA. Ketoprofen in oral surgery pain: a review. J Clin Pharmacol 1988; 28 (12 Suppl.): S40–6
Caldwell J, Hutt AJ, Fournel-Gigleux S. The metabolic chiral inversion and dispositional enantioselectivity of the 2-arylpropionic acids and their biological consequences. Biochem Pharmacol 1988; 37: 105–14
Hayball PJ, Nation RL, Bochner F. Enantioselective pharmacodynamics of the nonsteroidal anti-inflammatory drug ketoprofen: in vitro inhibition of human platelet cyclo-oxygenase activity. Chirality 1992; 4: 484–7
Avouac B, Teule M. Ketoprofen: the European experience. J Clin Pharmacol 1988; 28 Suppl. 12: S2–7
McCormack K, Urquhart E. Correlation between nonsteroidal anti-inflammatory drug efficacy in a clinical pain model and the dissociation of their anti-inflammatory and analgesic properties in animal models. Clin Drug Invest 1995; 9: 88–97
Palomer A, Cabré M, Ginesta J, et al. Resolution of racketoprofen esters by enzymatic reactions in organic media. Chirality 1993; 5: 320–8
Carganico G, Mauleón D, García ML. A novel arylpropionic derivate, its method of preparation and its application as an analgesic. (Spanish) Patent WO-94/11332 (Prior. ES 9202260, Nov-10-1992).
Williams RL, Upton RA. The clinical pharmacology of ketoprofen. J Clin Pharmacol 1988; 28 (12 Suppl.): S13–22
Jamali F, Brocks DR. Clinical pharmacokinetics of ketoprofen and its enantiomers. Clin Pharmacokinet 1990; 19: 197–217
Foster RT, Jamali F, Russell AS, et al. Pharmacokinetics of ketoprofen enantiomers in healthy subjects following single and multiple doses. J Pharm Sci 1988; 77: 70–3
Foster RT, Jamali F, Russell AS, et al. Pharmacokinetics of ketoprofen enantiomers in young and elderly arthritic patients following single and multiple doses. J Pharm Sci 1988; 77: 191–5
Foster RT, Jamali F, Russell AS. Ketoprofen enantiomers in synovial fluid. J Pharm Sci 1989; 78: 881–2
Foster RT, Jamali F, Russell AS. Pharmacokinetics of ketoprofen enantiomers in cholecsystectomy patients: influence of probenecid. Eur J Clin Pharmacol 1989; 37: 589–94
Stiegler S, Birkel M, Jost V, et al. Pharmacokinetics and relative bioavailability after single dose administration of 25 mg ketoprofen solution as compared to tablets. Methods Find Exp Clin Pharmacol 1995; 17: 129–34
Geisslinger G, Menzel S, Wissel K, et al. Pharmacokinetics of ketoprofen enatiomers after different doses of the racemate. Br J Clin Pharmacol 1995; 40: 73–5
Mauleón D, Artigas R, García ML, et al. Preclinical and clinical development of dexketoprofen. Drugs 1996; 52 Suppl. 5: 24–46
Sallustio BC, Abas A, Hayball PJ, et al. Enantiospecific highperformance liquid chromatography analysys of 2-phenylpropionic acid, ketoprofen and fenoprofen. J Chromtogr Biomed Appl 1986; 374: 329–37
Björkman S. Determination of enantiomers of ketoprofen in blood plasma by ion-pair extraccion and high-performance liquid chromatography of leucinamide derivates. J Chromtogr Biomed Appl 1987; 414: 465–71
Foster RT, Jamali F. High-performance liquid chromatography assay of ketoprofen enantiomers in humane plasma and urine. J Chromtogr Biomed Appl 1987; 416: 388–93
Menzel-Soglowek S, Geisslinger G, Brune K. Stereoselective high-performance liquid chromatography determinacion of ketoprofen, ibuprofen and fenoprofen in plasma using a chiral α1-acid glycoprotein column. J Chromtogr Biomed Appl 1990; 532: 295–303
Oda Y, Asakawa N, Abe S, et al. Avidin protein-conjugated column for injection analysis of drug enantiomers in plasma by high-performance liquid chromatography. J Chromtogr Biomed Appl 1991; 570: 133–41
Hayball PJ, Nation RL, Bochner F, et al. Enantiospecific analysis of ketoprofen in plasma by high-performance liquid chromatography. J Chromtogr Biomed Appl 1991; 570: 446–52
Palylyk E, Jamali F. Simultaneous determination of ketoprofen enantiomers and probenecid in plasma and urine by high-performance liquid chromatography. J Chromtogr Biomed Appl 1991; 568: 187–96
Oda Y, Asakawa N, Yoshida Y, et al. On-line determination and resolution of the enantiomers of ketoprofen in plasma using couple achiral-chiral high-performance liquid chromatography. J Pharm Biomed Anal 1992; 10: 81–7
Wright MR, Jamali F. Limited extent of stereochemical conversion of chiral non-steroidal anti-inflamatory drugs induced by derivatization methods employing ethyl chloroformate. J Chromatogr B Biomed Appl 1993; 616: 59–65
Lovlin R, Vakily M, Jamali F. Stereoselective high-performance liquid chromatography analysis of ketoprofen and its acyl glucuronides in chonic renal insufficiency. J Chromatogr B Biomed Appl 1996; 679: 196–8
Grubb NG, Rudy DW, Hall SD. Stereoselective high-performance liquid chromatography analysis of ketoprofen and its acyl glucuronides in chronic renal insufficiency. J Chromatogr B Biomed Appl 1996; 678: 237–44
Boisvert J, Caille G, McGliveray IJ, et al. Quantification of ketoprofen enantiomers in human plasma based on solid-phase extraction and enantioselective column chromatography. J Chromatogr B Biomed Sci Appl 1997; 690: 189–93
Eichhold TH, Bailey RE, Tanguay SL, et al. Determination of (R)- and (S)-ketoprofen in human plasma by liquid chromatography/tandem mass spectrometry following automated solid-phase extraction in the 96-well format. J Mass Spectrum 2000;35:504–11
Carr RA, Caillé G, Ngoc AH, et al. Stereospecific high-performance liquid chromatography assay of ketoprofen in human plasma and urine. J Chromatogr B Biomed Appl 1995; 668: 175–81
Jacks DS, Rumble RH, Davies NW, et al. Enantiospecific gas chromatographic-mass spectrometric procedure for the determination of ketoprofen and ibuprofen in synovial fluid and plasma: application to protein binding studies. J Chromtogr Biomed Appl 1992; 584: 189–97
Haginaka J, Murashima T, Fujima H, et al. Direct injection assay of drug enantiomers in serum on ovomucoid bonded silica material by liquid chromatography. J Chromatogr B Biomed Appl 1993; 620: 199–204
Ameyibor E, Stewart JT. HPLC determination of ketoprofen enantiomers in human serum using a nonporous octadecylsilane 1.5 microns column with hydroxypropyl beta-cyclodextrin as mobile phase additive. J Pharm Biomed Anal 1998; 17: 83–8
Davies NM. Methods of analysis of chiral non-steroidal anti-inflammatory drugs. J Chromatogr B Biomed Sci Appl 1997; 691: 229–61
Mcdowall RD, Pearce JC, Murkitt GS. Liquid-solid sample preparation in drug analysis. J Pharm Biomed Anal 1986; 4: 3–21
Jamali F, Mehvar R, Pasutto FM. Enantioselective aspects of drug action and disposition: therapeutic pitfalls. J Pharm Sci 1989; 78: 695–715
Barbanoj MJ, Gich I, Artigas R, et al. Pharmacokinetics of dexketoprofen trometamol in healthy volunteers after single and repeated oral doses. J Clin Pharmacol 1998; 38 (12 Suppl.): 33–40S
Gich I, Barbanoj MJ, Artigas R, et al. New fast-onset oral formulation of desketoprofen [abstract]. 6th Interscience World Conference on Inflammation, Antirheumatics, Analgesics and Immunomodulators (INWIN = 95); 1995 Mar 28–30; Geneva
McEwen J, De Luca M, Casini A, et al. The effect of food and an antiacid on the bioavailability of dexketoprofen trometamol. J Clin Pharmacol 1998; 38 (12 Suppl.): 41–5S
Gich I, Bayes M, Barbanoj MJ, et al. Bioinversion of R(−)-ketoprofen following oral administration in healthy volunteers. Clin Drug Invest 1996; 11: 347–53
Hayball PJ, Nation RL, Bochner F, et al. The influence of renal function on the enantioselective pharmacokinetics and pharmacodynamics of ketoprofen in patients with rheumatoid arthritis. Br J Clin Pharmacol 1993; 36: 185–93
Sallustio BC, Purdie YJ, Whitehead AG, et al. The disposition of ketoprofen enantiomers in man. Br J Clin Pharmacol 1988; 26: 765–70
Skeith KJ, Russell AS, Jamali F. Ketoprofen pharmacokinetics in the elderly: influence of rheumatic disease, renal function, and dose. J Clin Pharmacol 1993; 33: 1052–9
Bannwarth B, Lapicque F, Netter P, et al. The effect of food on the systemic availability of ketoprofen. Eur J Clin Pharmacol 1988; 33: 643–5
Jamali F, Mathers D, Russell AS. Concentration-effect relationship of 5-ketoprofen in adjuvant arthritic rats. Pharm Res 1989; 6 Suppl.:S211
Orme MLE. The relationship between the plasma concentration of non-steroidal anti-inflammatory drugs and their therapeutic effect. Agents Actions 1985; 17: 151–5
Muller N, Payan E, Lapicque F, et al. Pharmacological aspects of chiral nonsteroidal anti-inflammatory drugs. Fundam Clin Pharmacol 1990; 4: 617–34
Zhivkova ZD, Russeva VN. Stereoselective binding of ketoprofen enantiomers to human serum albumin studied by high-performance liquid affinity chromatography. J Chromatogr B Biomed Appl 1998; 714: 277–83
Hayball PJ, Nation RL, Bochner F, et al. Plasma protein binding of ketoprofen enantiomers in man: method development and its application. Chirality 1991; 3: 460–6
Dubois N, Muller N, Lapicque F, et al. Stereoselective protein binding of nonsteroidal anti-inflammatory drugs: pharmacological consequences. Therapie 1993; 48: 335–9
Dubois N, Lapicque F, Abiteboul M, et al. Stereoselective protein binding of ketoprofen: effect of albumin concentration and of the biological system. Chirality 1993; 5: 126–34
Sakai T, Maruyama T, Sako T, et al. Stereoselective serum protein binding of ketoprofen in liver diseases. Enantiomer 1999; 4: 477–82
Sakai T, Maruyama T, Imamura H, et al. Mechanism of stereoselective serum binding of ketoprofen after hemodialysis. J Pharmacol Exp Ther 1996; 278: 786–92
Wallis WJ, Simkin PA. Antirheumatic drug concentrations in human synovial fluid and synovial tissue. Clin Pharmacokinet 1983; 8: 496–522
Fears S. Lipophilic xenobiotic conjugates: the pharmacological and toxicological consequences of the participation of drugs and other foreign compounds as substrates in lipid biosynthesis. Prog Lipid Res 1985; 24: 177–95
Caldwell J. Xenobiotic acyl-coenzymes A: critical intermediates in the biochemical pharmacology and toxicology of carboxylic acids. Biochem Soc Trans 1984; 12: 9–11
Williams K, Day R, Knihinicki R, et al. The stereoselective uptake of ibuprofen enantiomers into adipose tissue. Biochem Pharmacol 1986; 35: 3403–5
Sallustio BC, Meffin PJ, Knights KM. The stereospecific incorporation of fenoprofen into rat hepatocycle and adipocyte triacylglycerols. Biochem Pharmacol 1988; 37: 1919–23
Zhao B, Geisslinger G, Hall I, et al. The effect of the enantiomers of ibuprofen and flurbiprofen on the beta-oxidation of palmitate in the rat. Chirality 1992; 4: 137–41
Knights KM, Drew R. The effects of ibuprofen enantiomers on hepatocyte intermediary metabolism and mitochondrial respiration. Biochem Pharmacol 1992; 44: 1291–6
Roberts BJ, Knights KM. Inhibition of rat peroxisomal palmitoyl-CoA ligase by xenobiotic carboxylic acids. Biochem Pharmacol 1992; 44: 261–7
Mayer JM, Roydevis M, Audergon C, et al. Interactions of anti-inflammatory 2-arylpropionates (profens) with the metabolism of fatty acids: in vitro studies. Int J Tissue React 1994; 16: 59–72
Morgan A, Clark D. CNS adverse effects of nonsteroidal anti-inflammatory drugs: therapeutic implications. CNS Drugs 1998;9:281–90
Carabaza A, Suesa N, Tost D, et al. Stereoselective metabolic pathways of ketoprofen in the rat: incorporation into triacylglycerols and enantiomeric inversion. Chirality 1996; 8: 163–72
Populaire P, Terlain B, Pascal S, et al. Biological behaviour: plasmatic levels, excretion and biotransformation of 2-(3-benzoylphenyl) propionic acid (ketoprofen) in animals and man. Ann Pharm Fr 1973; 31: 735–49
Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 RP) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976; 14: 45–52
Iwakawa S, He X, Hashimoto S, et al. Stereoselective disposition of ketoprofen in rats. Drug Metab Dispos 1991; 19:717–8
Foster RT, Jamali F. Stereoselective pharmacokinetics of ketoprofen in the rat. Influence of route of administration. Drug Metab Dispos 1998; 16: 623–6
Aberg G, Ciofalo VB, Pendleton RG, et al. Inversion of (R)- to (S)-ketoprofen in eight animal species. Chirality 1995; 7: 383–7
Jamali F. Pharmacokinetics of enantiomers of chiral nonsteroidal anti-inflammatory drugs. Eur J Drug Metab Pharmacokinet 1988; 13: 1–9
Mayer JM. Stereoselective metabolism of anti-inflammatory 2-arylpropionates. Acta Pharm Nord 1990; 2: 197–216
Wechter WJ. Drug chirality: on the mechanism of R-aryl propionic acid class NSAIDs. Epimerization in humans and the clinical implications for the use of racemates. J Clin Pharmacol 1994; 34: 1036–42
Nakamura Y, Yamaguchi T, Takahashi S, et al. Optical isomerization mechanism of R-(−)-hydratropic acid derivatives. J Pharmacobio Dyn 1980; 3(5): S–1
Reichel C, Bang H, Brune K, et al. 2-arylpropionyl-CoA epimerase: partial peptide sequences and tissue localization. Biochem Pharmacol 1995; 50: 1803–6
Benoit E, Delatour P, Olivier L, et al. (−)-R-fenoprofen: formation of fenoprofenyl-coenzyme A by rat liver microsomes. Biochem Pharmacol 1995; 49: 1717–20
Soraci A, Benoit E. In vitro fenoprofenyl-coenzyme A thioester formation: interspecies variations. Chirality 1995; 7: 534–40
Jamali F, Russell AS, Foster RT, et al. Ketoprofen pharmacokinetics in humans: evidence of enantiomeric inversion and lack of interacion. J Pharm Sci 1990; 79: 460–1
Mis R, Tost D, Ortega E, et al. Bioinversion of ketoprofen enantiomers in several species [abstract]. Methods Find Exp Clin Pharmacol 1994; 16 Suppl. 1:81
Jamali F, Lovlin R, Aberg G. Bi-directional chiral inversion of ketoprofen in CD-1 mice. Chirality 1997; 9: 29–31
Rudy AC, Liu Y, Brater C, et al. Stereoselective pharmacokinetics and inversion of (R)-ketoprofen in healthy volunteers. J Clin Pharmacol 1998; 38 (12 Suppl.): 3–10S
Rollins DE, Klaasseen CD. Biliary excretion of drugs in man. Clin Pharmacokinet 1979; 4: 368–79
Nation RL. Chirality in new drug development: clinical pharmacokinetic considerations. Clin Pharmacokinet 1994; 27: 249–55
Ghezzi P, Melillo G, Meazza C, et al. Differential contribution of R and S isomers in ketoprofen anti-inflammatory activity: role of cytokine modulation. J Pharmacol Exp Ther 1998; 287: 969–74
Grubb NG, Rudy DW, Brater DC, et al. Stereoselective pharmacokinetics of ketoprofen and ketoprofen glucuronide in end-stage renal disease: evidence for a ‘futile cycle’ of elimination. Br J Clin Pharmacol 1999; 48: 494–500
Bannwarth B, Lagrange F, Péhourcq F, et al. (S)-ketoprofen accumulation in premature neonates with renal failure who were exposed to the racemate during pregnancy. Br J Clin Pharmacol 1999; 47: 459–61
Dubois N, Lapicque F, Maurice MH, et al. In vitro irreversible binding of ketoprofen glucuronide to plasma proteins. Drug Metab Dispos 1993; 21: 617–23
Cabre F, Ferna’ndez MF, Zapatero MI, et al. Intestinal ulcerogenic effect of S(+)-ketoprofen in the rat. J Clin Pharmacol 1998; 38 (12 Suppl.): 27S–32S
Davies NM, Wright MR, Russell AS, et al. Effect of the enantiomers of flurbiprofen, ibuprofen, and ketoprofen on intestinal permeability. J Pharm Sci 1996; 85: 1170–3
Kommuro TR, Khan MA, Reddy IK. Racemate and enantiomers of ketoprofen: phase diagram, thermodynamic studies, skin permeability, and use of chiral permeation enhancers. J Pharm Sci 1998; 87: 833–40
Alvarez C, Torrado JJ, Cadorrniga R. Stereoselective drug release from ketoprofen and ricobendazole matrix tablets. Chirality 1999; 11: 611–5
Gay C, Planas E, Donado M, et al. Analgesic effect of low doses of dexketoprofen in the dental pain model: a randomised, double-blind, placebo-controlled study. Clin Drug Invest 1996; 11:320–30
McGurk M, Robinson P, Rajayogeswaran V, et al. Clinical comparison of dexketoprofen trometamol, ketoprofen, and placebo in postoperative dental pain. J Clin Pharmacol 1998; 38 (12 Suppl.): 46S–54S
Ezcurdia M, Cortejoso FJ, Lanzón R, et al. Comparison of the efficacy and tolerability of dexketoprofen and ketoprofen in the treatment of primary dysmenorrhea. J Clin Pharmacol 1998;38:65S–73S
Beitra’n J, Martín-Mola E, Figueroa M, et al. Comparison of dexketoprofen trometamol and ketoprofen in the treatment of osteoarthritis of the knee. J Clin Pharmacol 1998; 38 (12 Suppl.): 74S–80S
Baga’n JV, López JS, Valencia E, et al. Clinical comparison of dexketoprofen trometamol and dipyrone in postoperataive dental pain. J Clin Pharmacol 1998; 38 (12 Suppl.): 55S–64S
Cooper SA, Reynolds DC, Reynolds B, et al. Analgesic efficacy and safety of (R)-ketoprofen in postoperative dental pain. J Clin Pharmacol 1998; 38 (12 Suppl.): 11S–8S
Wecheter WJ. Dexketoprofen trometamol [editorial]. J Clin Pharmacol 1998; 38 (12 Suppl.): 1S–2S
Forbes AJ. Oral surgery. In: Max MB, Portenoy RK, Laska EM, editors. Advances in pain research and therapy. Vol 18. The design of analgesic clinical trials. New York: Raven Press, 1991: 347–74
Cooper S, Beaver WT. A model to evaluate mild analgesics in dental pain. Clin Pharmacol Ther 1976; 20: 241–50
Swedberg JA, Steinbauer JR. Osteoarthritis. Am Fam Physician 1992; 45: 557–68
Dawood MY. Dysmenorrhea. In: Max MB, Portenoy RK, Laska EM, editors. Advances in pain research and therapy. Vol 18. The design of analgesic clinical trials. New York: Raven Press, 1991:429–43
Cooper S, Gelb SB, Maggio Cavaliere MB, et al. An analgesic relative potency assay comparing ketoprofen and aspirin in postoperative dental pain. Adv Ther 1984; 1: 410–8
Woolf CJ. A new strategy for the treatment of inflammatory pain. Prevention or elimination of central sensitization. Drugs 1994; 47 Suppl. 5: 1–9
McCormack K. The spinal actions of nonsteroidal anti-inflammatory drugs and the dissociation between their anti-inflammatory and analgesic effects. Drugs 1994; 47 Suppl. 5: 28–45
McCormack K. Non-steroidal anti-inflammatory drugs and spinal nociceptive processing. Pain 1994; 59: 9–43
Caschman J, McAnulty G. Nonsteroidal anti-inflammatory drugs in perisurgical pain management: mechanism of action and rationale for optimum use. Drugs 1995; 49: 51–70
Geisslinger G, Ferreira SH, Menzel S, et al. Antinociceptive actions of R(−)-flurbiprofen: a non-cyclooxygenase inhibiting 2-arylpropionic acid in rats. Life Sci 1994; 54: 173–7
Lotsch J, Geisslinger G, Mohammadian P, et al. Effects of flubiprofen enantiomers on pain-related chemosomatosensory evoked potentials in human subjects. Br J Clin Pharmacol 1995; 40: 339–46
Geisslinger G, Schaibe HG. New insights into the site and mode of antinociceptive action of flurbiprofen enantiomers. J Clin Pharmacol 1996; 36: 513–20
Acknowledgements
The authors thank Cristina Moros and Mercedes Yritiar for their invaluable collaboration in the reference search performed, as well as Miss Ma Angeles Funes for her collaboration in the final typed version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbanoj, MJ., Antonijoan, RM. & Gich, I. Clinical Pharmacokinetics of Dexketoprofen. Clin Pharmacokinet 40, 245–262 (2001). https://doi.org/10.2165/00003088-200140040-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200140040-00002