Article Text
Abstract
Objectives Assess the 12-month efficacy of a brief intervention (BI) on reducing drug use and increasing drug treatment services utilisation among adult emergency department (ED) patients.
Methods This randomised, controlled trial enrolled 18–64-year-old ED patients needing a drug use intervention. Treatment arm participants received a tailored BI while control arm participants only completed the study questionnaires. Self-reported past 3-month drug use and engagement in drug treatment services were compared by study arm at 3-month intervals over 1 year. Multiple imputations were performed to overcome loss-to-follow-up.
Results Of the 1030 participants, follow-up completion ranged 55%–64% over the four follow-ups. At 12 months, the two study arms were similar in regards to mean: (1) proportion reporting any drug use (treatment: 67.1% (61.6 to 72.6), control: 74.4% (69.4 to 79.4)); (2) drug use frequency on a five-point scale (treatment: 3.7 (3.3 to 4.2), control: 4.6 (4.0 to 5.2)); (3) total days of drug use (treatment: 28.3 (23.2 to 33.4), control: 33.4 (28.5 to 38.2)); (4) most number of times drugs used/day (treatment: 4.6 (3.6 to 5.5), control: 6.1 (4.8 to 7.3)) and (5) typical number of times drugs used/day (treatment: 3.3 (2.5 to 4.1), control: 5.1 (3.9 to 6.2)). Utilisation of drug treatment services also was similar by study arm. In multivariable regression analyses, patients who were homeless or had higher drug use at baseline continued to have greater drug use in follow-up.
Conclusions Among adult ED patients requiring a drug use intervention, this BI did not decrease drug use or increase drug treatment services utilisation over a 12-month period more than the control condition.
Trial registration number NCT01124591; Pre-trial.
- drug abuse
- mental health, drug abuse
Statistics from Altmetric.com
Key messages
What is already known on this subject
Although EDs frequently provide care to adult patients who use drugs, efficacious methods of reducing or eliminating drug use among our patients continue to be elusive. In a previous publication on the short-term results of a randomised, controlled trial, we reported that a brief intervention (BI) did not reduce drug use or increase treatment services utilisation more than the control condition. The long-term effects of this programme could be different.
What this study adds
In this 12–month prospective cohort study conducted in USA, reported drug use, frequency of drug use, days of use, most times used/day and typical times used/day were not reduced among adult ED patients receiving the BI compared with controls. Drug treatment services utilisation was infrequent among all study patients and was not greater among patients who received a BI. Our findings suggest that other approaches to decreasing drug use are needed.
Introduction
Despite screening, brief intervention and referral to treatment (SBIRT) models demonstrating some efficacy in reducing alcohol1–6 and marijuana use7 8 among ED patients, recent ED-based brief interventions (BIs) have not shown benefit for patients who use drugs.9 10 If BIs designed to reduce or eliminate drug use are not effective, other approaches are needed to impact this serious public health problem. We recently reported the short-term results of a randomised, controlled trial Brief Intervention for Drug Misuse for the Emergency Department (BIDMED) that assessed the efficacy of a BI aimed at decreasing drug use and increasing drug treatment services utilisation among adult ED patients.11 While self-reported drug use decreased in both study arms over a 3-month period, the BI did not reduce drug use or increase treatment services utilisation more than the comparison condition. However, it is possible that the BI might have delayed effects beyond the short-term period. For example, following a BI, an individual might not significantly reduce drug use until after completing a treatment programme and avoiding the environment that facilitates drug use. The shortage of drug use treatment programmes across USA can contribute to the delay in effect after the BI. Furthermore, drug use patterns do not necessarily exhibit a steady pattern; drug use may increase or decrease over time, which indicates a need for a longer longitudinal assessment. In addition, the true value of any intervention is in its relative permanence, rather than short-term changes.
The primary aim of this investigation was to determine if the BI was more efficacious than no BI in reducing any drug use (illicit or prescription drug use) over a 12-month period. The secondary aim was to assess the efficacy of this BI as compared with no BI in increasing utilisation of drug treatment services over this 1-year period. The third aim was to identify patients who might benefit from the BI.
Methods
Study design and setting
Details about the BIDMED randomised, controlled trial development and conduct have been published previously.11 12 Key points are summarised for this manuscript. BIDMED enrolled participants from July 2010 to December 2012 at two urban EDs (The Miriam Hospital and Rhode Island Hospital in Providence, Rhode Island, USA). The trial was registered prospectively (ClinicalTrials.gov identifier: NCT01124591). We obtained a certificate of confidentiality from the National Institutes of Health due to the sensitive nature of the drug use questions and informed study participants about its potential protections and limitations.
Selection of participants
Data collection for BIDMED was performed from 08:00 am to midnight 7 days/week when bilingual (English-speaking and Spanish-speaking) research assistants (RAs) were available to conduct the study. Using an online, random number generator (http://www.random.org), RAs selected a random sample of adult patients present in the ED during study collection periods and reviewed their ED electronic medical record for study eligibility. The RAs would briefly interview potential participants to confirm study eligibility.
Patients were study eligible if they were 18–64 years old; English-speaking or Spanish-speaking; not critically ill or injured; not prison inmates, under arrest or undergoing home confinement; not presenting for an acute psychiatric illness; not requesting treatment for substance use/misuse; not intoxicated and did not have a physical or mental impairment that prevented them from providing consent or participating in the study. The inclusion/exclusion criteria purposely included ED patients who would be targeted for screening for drug abuse in an ED SBIRT programme and excluded patients expected to be evaluated and treated for drug abuse in usual ED practice (ie, presenting for drug treatment, a psychiatric problem, intoxicated, presenting after a drug overdose).
Potential study-eligible ED patients completed the Alcohol, Smoking and Substance Involvement Screening Test, Version 3 (ASSIST V.3) via audio computer self-administered interviewer (ACASI). They also reported the estimated total number of days that they had used drugs in the previous 3 months, the maximum number of drugs used per day and the typical number of drugs that they had used per day (online supplementary material for the English-language study questionnaire). Per WHO recommendations for the need of at least a BI for drug use, ED patients with an ASSIST score of ≥4 points for any drug category or ever injected drugs were invited to enrol in BIDMED.13 ASSIST scores≥27 points for any drug category by WHO criteria indicate the need for a more intensive intervention. The need for an intensive intervention at enrolment was accounted for in the study analysis.
Supplementary file 1
Methods and measurements
Following enrolment, participants were randomly assigned 1:1 into the two study arms (treatment vs control) using block randomisation with a block size of six. RAs were blinded to the randomisation block size, study arm designation (treatment vs control) until random assignment was made and participants had responded, hence were unable to affect study arm designation. Afterwards, participants completed the rest of the study questionnaires via the ACASI, including the Treatment Services Review questionnaire, which measured utilisation of drug treatment services.14 Participants completed the follow-up questionnaires every 3 months for up to 1-year post-ED enrolment. Participants completed the baseline and follow-up study questionnaires in approximately 10–15 min each and received a gift card to a local store.
Interventions
Participants randomly assigned to the treatment arm underwent a 20–30 min BI designed to facilitate behaviour change with a RA trained in motivational interviewing techniques (see online supplementary material).15 This training consisted of 8 hours of general instruction on motivational interviewing theory and practice, demonstrations and role-playing with a Motivational Interviewing Network of Trainers (MINT)-certified trainer. In addition, the RAs engaged in 40 hours of training specific to the BIDMED BI, which involved review of the protocol, demonstrations and role-playing under supervision of the study author psychologists. The primary goal of the BI was to motivate participants to reduce their drug misuse and seek appropriate treatment. The BI sessions were based on motivational interviewing16 and the health beliefs model.17 BI arm participants were contacted via telephone for a booster session by the same RA 2–4 weeks post-ED enrolment. Fidelity to the BI was monitored through review of voice recordings of the BI sessions, which were discussed with the RAs weekly and suggestions for improvement were provided.
Supplementary file 2
Outcomes
The primary outcome measured was reduction in self-reported total drug use during the prior 3 months at each follow-up, as measured by the study questionnaires. Total drug use was the summation of all 12 drug categories assessed (eg, cocaine, benzodiazepines, prescription opioids). The primary outcome was stratified by self-reported marijuana use, since marijuana was the most frequently reported drug used. The secondary outcome was self-reported utilisation of drug treatment services within the past 3 months per the Treatment Services Review. The third outcome aimed to identify subgroups of participants who might benefit more or less from the BI. Outcomes were assessed every 3 months over a 12-month period. We based the sample size needed for BIDMED on the primary aim of a hypothesised 25% greater decrease in drug use in the treatment versus the control arm at 12 months, based on the work from prior researchers.18–20 Per this hypothesis, we estimated a needed sample size of 550 per study arm for an 80% power and a two-sided Type I error rate of 0.05, assuming a loss to follow-up rate of 20% at 12 months post-recruitment.
Analysis
Study eligibility assessments and enrolment were summarised using the CONSORT approach.21 We compared the baseline demographic characteristics and self-reported substance use by study arm for those enrolled and for those who completed or did not complete each follow-up using summary statistics with corresponding 95%CIs. As would be expected in longitudinal studies, particularly for drug use research follow-up studies, the data were incomplete due to participants not completing one or more of their required follow-up questionnaires. Per recommended methods,22 we performed multiple imputations procedures using conditional models that used data from prior follow-ups to impute missing values for subsequent follow-ups, which is a sequential strategy that mirrors the longitudinal, prospective nature of the study (eg, imputations of 9-month follow-up responses were conditional on 6-month, 3-month and baseline responses). We employed fully conditional specification methods to impute the missing data since some missing values were continuous and others were categorical.23 We assumed that data were missing at random, conditional on the covariates for study arm, baseline demographic characteristics, baseline ASSIST scores, receipt of a booster call and baseline and prior follow-up questionnaire responses. The multiple imputations procedures created 25 imputed datasets for the entire study period.24 The imputation steps were followed by imputation model diagnostics that involved comparing the means, SD and histograms of the imputed data to the observed data and then by monitoring the Monte Carlo Markov Chains while the imputation models were processing to check for lack of convergence.25 Both diagnostic procedures indicated that the imputation models performed satisfactorily. Through these aforementioned processes, we created 25 imputed cases datasets that were used for the subsequent analyses.
Using the 25 imputed cases datasets, the primary outcome of reduction in self-reported drug use/misuse within the prior 3 months at each follow-up was compared by study arm, as follows: (1) proportions of participants reporting any drug use at each follow-up; (2) frequency of drug use; (3) total days of drug use; (4) most number of drugs used/day and (5) typical number of drugs used/day. Frequency of drug use was reported on a five-point response scale that ranged from no drug use to near-daily/daily drug use (5–7 days/week) in the past 3 months. For the secondary outcome of drug treatment services utilisation, we compared study arms by those who sought or received treatment within the prior 3 months at each follow-up. To assess the consistency of the study findings, the analyses were repeated using two separate datasets; one database contained participants who completed all four follow-ups (completed cases), while the other contained participants who completed any of the follow-ups (available cases). Using the 25 imputed cases datasets, we created graphs depicting the primary and secondary outcomes over time. Each point estimate constituted the mean of the outcome value from the 25 imputed datasets with corresponding 95% confidence bands to indicate differences between study arms.
We conducted a longitudinal assessment of the treatment effect of the BI on the primary and secondary outcomes over the 12-month study period using the imputed cases dataset. This assessment also served to identify patients who might benefit from the BI. We constructed generalised linear models (GLMs) that adjusted for demographic characteristics, baseline ASSIST scores and receipt of a booster call. Generalised estimating equations (GEEs) with first-order, autoregressive, within-subject correlational structure were employed to account for the repeated measures nature of the data. The 25 imputed datasets were analysed via GLMs and GEEs, and the pooled values for the beta coefficients (β) and corresponding 95% CIs were estimated. All analyses were conducted using STATA V.13.1 and SAS V.9.4.
Results
Participant enrolment, characteristics and retention
As shown in figure 1, the four 3-month follow-up rates ranged from approximately 55%–64%. Of those in the treatment arm (n=516), 37% completed all four follow-ups, while 17% completed one, 15% two, 10% three and 21% did not complete any follow-ups. Of those in the control arm (n=514), 40% completed all four follow-ups, while 19% completed one, 12% two, 10% three and 19% did not complete any follow-ups. The demographic characteristics (table 1) and self-reported substance use (table 2) of those who enrolled in the study (baseline) were similar between study arms. At baseline, the proportion of participants who ever had received drug misuse treatment also was similar (35.5% ((control)) vs 36.6% (treatment); difference: −1.1% (95% CI −6.0 to 8.7%). Online supplementary tables 1 and 2 provide the demographic characteristics of the completed cases (participants who completed questionnaires at baseline and all subsequent follow-ups) and the available cases (participants who completed questionnaires at baseline and at least one of the follow-ups) across all follow-ups. The demographic characteristics and self-reported substance use of those who completed versus did not complete each follow-up were also similar (online supplementary tables 3 and 4).
Supplementary file 3
Eligibility assessment and enrolment and follow-up of participants.
Comparison of demographic characteristics of participants at study enrolment by study arm
Comparison of past 3-month reported drug use at study enrolment
Reduction in drug use/misuse
As shown in figure 2 using the imputed dataset that attempted to account for loss-to-follow-up, there were no differences between the two study arms at all follow-ups for the primary outcome of a reduction in self-reported past 3-month total drug use. At 12 months, the two study arms were similar in regards to mean: (1) proportion reporting any drug use (treatment: 67.1% (61.6 to 72.6), control: 74.4% (69.4 to 79.4)); (2) drug use frequency on a five-point scale (treatment: 3.7 (3.3 to 4.2), control: 4.6 (4.0 to 5.2)); (3) total days of drug use (treatment: 28.3 (23.2 to 33.4), control: 33.4 (28.5 to 38.2)); (4) most number of times drugs used/day (treatment: 4.6 (3.6 to 5.5), control: 6.1 (4.8 to 7.3)) and (5) typical number of times drugs used/day (treatment: 3.3 (2.5 to 4.1), control: 5.1 (3.9 to 6.2)). These findings were similar for total drug use including or excluding marijuana use (figure 2). In the longitudinal GEE model analyses (table 3), the regression coefficient (β) represents the difference between the treatment and control groups over the 12-month study period. As an example of interpreting the β coefficients as differences in this context, the average number of times of drugs were typically used per day (excluding marijuana) was 0.1 times/day higher in the treatment group than the control group. However, this difference did not reach statistical significance. For all regression coefficients, there also were no differences between study arms over the entire 12-month follow-up period for the primary outcome. Analyses of the complete cases and the available cases yielded similar results (online supplementary tables 5–8).
Comparison of drug use outcome at each follow-up (imputed cases).
Longitudinal assessment of impact of treatment effect on drug use outcomes over the 12-month study period (imputed cases)
Increase in drug treatment services utilisation
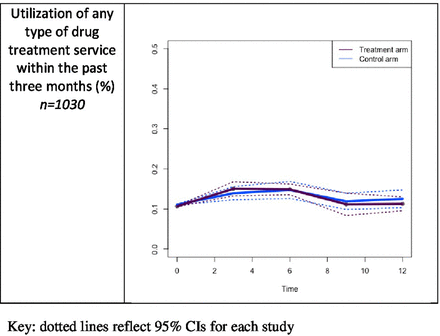
Using the imputed dataset, there were no differences between the study arms in the proportions of participants who sought or received drug treatment services within the prior 3 months at each follow-up (figure 3). The results of the longitudinal GEE model analyses also indicate no differences between study arms over the 12-month follow-up period for drug treatment services utilisation. These results were similar for the complete cases (β 0.2 (−0.5 to 0.9)) and available cases (β −0.3 (−0.6 to 0.0)).
Comparison of percentage of participants using any treatment service for drug use at each follow-up (imputed cases).
Demographic and clinical variations in response to the BI
In the multivariable GEE models adjusting for study arm, participant demographic characteristics, baseline drug misuse severity (per the ASSIST scores) and receipt of a booster call, there were no identifiable factors associated with a greater predilection to benefit from the BI in regards to reduction of total drug use (online supplementary table 9) using the imputed dataset. However, greater frequency of drug use was related to higher drug use at baseline (per the ASSIST scores) and homelessness.
Discussion
The disappointing results of the BI used in this longitudinal, randomised, controlled trial aimed to reduce or eliminate drug use are echoed by other ED-based and outpatient clinic investigations.9 10 26 27 Woodruff et al observed that among approximately 700 San Diego ED patients randomly assigned to a BI on drug use versus a BI on safer driving practices, there were no differences in past 30-day drug use abstinence at 6 months post-enrolment.9 Bogenschutz et al also observed that among 1285 adult ED patients across six recruitment sites, participants randomly assigned to a BI with telephone boosters as compared with screening, assessment and referral to treatment or minimal screening did not report better drug use at 3, 6 and 12 months post-enrolment.10 It is apparent from this study and these other investigations that other approaches are needed.
Even though the reasons cannot be ascertained for the failure of the BI used in this investigation in reducing drug use/misuse by the results of this trial, we can speculate on some possibilities. First, the particular approach, content, format or delivery of the BI used in this study might not be appropriate for the needs of the patient population. Approaches other than motivational interviewing, content more likely to induce behavioural change, format other than one-on-one discussions and delivery perhaps by someone other than a RA (eg, a drug use counsellor or a peer navigator) can be investigated in future studies. Second, BIs might be inadequate because of their brevity. A sustained, longitudinal intervention involving greater contact might be required. Third, a ‘one-size fits’ all approach to offering BI to the wide variety of drugs that ED patients illicitly and harmfully use might work only for particular drugs. Fourth, although less likely, given the null effects of our study and others,9 10 26 27 the population studied, the setting and problems of lack of follow-up also could account for the BI failure. Fifth, utilisation of drug treatment programs post-ED enrolment among study participants in this investigation was infrequent; higher utilisation might have led to better observed outcomes. Sixth, pairing BIs with pharmacological treatment also might have led to better results.
Our study had several limitations. As we acknowledged in our previous publication,11 these limitations include: (1) potential for lack of external validity to patients excluded from the study; those with different substance use, socioeconomic and demographic characteristics; those with greater or lesser access to drug treatment services and who presented during the overnight hours when data collection was not performed; (2) inability to confirm drug use reduction and treatment services utilisation; (3) potential for exaggerated or false self-reporting; (4) possibility that the study instruments were not ideal for the outcomes measured; (5) potential for different results if the interventions were performed by clinicians with greater experience in substance use interventions; (6) inability to determine if the intervention might be more successful for particular drugs and (7) the sample size might have been inadequate to detect small effects.
One of the most important limitations of the study is the relatively large loss-to-follow-up, which has been observed in other studies involving substance misusing populations, who comprise a socioeconomically disadvantaged, highly mobile and unstably housed population.4 However, limiting the study population at enrolment to those who might have been more easily tracked could have reduced the external validity of the study results. Although we employed accepted approaches to accounting for missing data, we cannot verify that these approaches were optimal, and unmeasured factors might have accounted for missingness and could not be adjusted for in our models. Imputation relies on the assumption that the data imputed mimics that of those who completed the follow-ups, which might be incorrect. Greater follow-up might have conferred different results.
In conclusion, in this randomised, controlled trial, the BI provided in this study did not decrease drug use or increase drug treatment services utilisation among adult ED patients more than the control condition over a 12-month period. Other approaches are needed to reduce the negative impact of this continued pervasive problem among ED patients.
Acknowledgments
The research team gratefully acknowledges the assistance of Vera Bernardino for preparing the data for analysis and publication, the research assistants who assessed patients for the study and helped coordinate the study (Naira Arellano, Vera Bernardino, Rosalie Berrios-Candelaria, Vianella Burgos, Ian Donaghy, Dora Estrela, Cindy Gonzalez, Alyssa Hozey, Michelle Leveillee, Stefanie Paolino, Ayanaris Reyes and Becca Rose) and the support of the staff and patients at our two hospitals. The authors gratefully acknowledge the assistance of Michael J Mello, MD, MPH and Ted Nirenberg, PhD.
References
Footnotes
Contributors RCM was the principal investigator of the investigation and primary author of the manuscript. He was involved in all stages of the manuscript composition. ZhoZ and ZihZ were involved in the data analysis and manuscript composition. TL was a study co-investigator and supervised the data analysis and contributed to the manuscript. JRB was a study co-investigator and assisted in the study conduct and in the preparation of the analyses and the resultant manuscript.
Funding This research was supported by grants from the National Institute on Drug Abuse (R01 DA026066) and the Lifespan/Tufts/Brown Centers for AIDS Research (P30 AI042853). ClinicalTrials.gov identifier: NCT01124591.
Competing interests None declared.
Patient consent Obtained.
Ethics approval Rhode Island Hospital Institutional Review Board.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data from the study are available from the study authors commensurate with appropriate data sharing uses and federal regulations.
Presented at Preliminary findings from this study were presented at the Society for Academic Emergency Medicine annual meeting in New Orleans, Louisiana on 12 May 2016.