Article Text
Abstract
Background Procedural sedation is a core skill of the emergency physician. Bolus administration of propofol is widely used in UK EDs. Titrated to an end point of sedation, it has a rapid effect but has been associated with adverse incidents. The use of a target-controlled infusion (TCI) of propofol is not routine but may reduce the incidence of adverse incidents.
The primary aims of this single-arm feasibility study were patient satisfaction and to establish recruitment rates for a randomised controlled trial comparing propofol TCI to bolus administration.
Methods Four EDs in Scotland, UK, participated. Patients aged 18-65 years, with anterior shoulder dislocation, weight ≥ 50kg, fasted ≥ 90 min were screened. Patients underwent reduction of their dislocated shoulder using TCI propofol. The primary end point was patient satisfaction recorded on a Visual Analogue Scale.
Results Between 3 April 2017 and 31 December 2018, 25 patients were recruited with a recruitment rate of 20% for the 16-month recruitment window, with a temporary pause to allow amendment of drug dosage.
Two patients were excluded. Twenty achieved adequate sedation, defined as a Modified Observer’s Assessment of Alertness/Sedation Scale (OAA/S) 3. Successful reduction was achieved in all adequately sedated. Patient satisfaction was documented in 14 patients, mean±SD of 97±9 and time to sedation was 25±8 min. No adverse events were recorded using the Society of Intravenous Anaesthesia adverse event reporting tool.
Conclusion Propofol TCI was acceptable as a method of procedural sedation for patients. The lower than expected recruitment rates highlight the need for dedicated research support.
Trial registration number NCT03442803.
- analgesia/pain control
- fractures and dislocations
- airway
- emergency department management
- safety
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Key messages
What is already known on this subject
Propofol is one of the most commonly used agents for procedural sedation in the ED.
Bolus administration of propofol has a rapid effect but is associated with adverse events such as loss of airway patency and hypotension.
Target-controlled infusion (TCI) allows for controlled titration and may reduce the incidence of adverse events.
What this study adds
In this multicentre, single-arm feasibility study, we found that propofol TCI is an acceptable method of procedural sedation in the ED patient population requiring reduction of an anterior shoulder dislocation.
Background
Procedural sedation has long been a core skill of the emergency physician. In recent years, the safety and efficacy of procedural sedation has been enhanced as newer sedative and analgesic agents have emerged and been adopted into clinical practice.1 In 2012, the Royal College of Emergency Medicine collaborated with the Royal College of Anaesthetists to publish guidelines on safe sedation in the ED.2 3 Despite this, concern regarding the safety of ED procedural sedation persists.4–8
The practice of using propofol to achieve sedation in the ED originated around the turn of the millennium,9 and has since become one of the most common choices of sedative in the ED.4 10 Propofol offers a number of advantages as a sedative agent, including a rapid onset and recovery time, amnesiac properties and good efficacy.11 12 It is commonly administered in repeat boluses of a few mLs at a time until the desired sedation effect is achieved. When administered as a bolus, the operator may be underdose, delivering an insufficient effect site concentration, or over-dose, exceeding the desired effect site concentration.
Target-controlled infusion (TCI) has been suggested as a potential solution to the adverse events experienced with the bolus administration of propofol. In contrast to bolus administration of propofol, TCI allows the operator to accurately target a specific clinical effect. TCI is widely used in anaesthetic practice,13–15 and has been studied in a number of settings including gastrointestinal endoscopy, dental surgery, oocyte retrieval8 and bronchoscopy.14 To our knowledge, propofol TCI has not been studied in an ED setting.
A feasibility study was needed to provide evidence that TCI propofol is acceptable to the patient and to provide information about recruitment including barriers to ensure that a future multicentre randomised controlled trial (RCT) can be adequately powered to show statistical significance. The incidence of adverse events using bolus versus TCI propofol in the ED will be the primary outcome measure in the future RCT.
Progression to a multicentre RCT will require evidence of the ability to adequately recruit and that it is an acceptable method of procedural sedation to the patient.
Methods
This multicentre, single-arm feasibility study was carried out in four EDs in the West of Scotland; three in busy urban hospitals and one in a district general hospital. Our study population was the adult patient (≥18 years), requiring sedation to facilitate the reduction of an acute traumatic anterior shoulder dislocation in the ED.
The study protocol16 was published in pilot and feasibility trial protocol (https://pilotfeasibilitystudies.biomedcentral.com/articles/10.1186/s40814-019-0412-y) studies.
Patients included were aged 18–65 years, weighed ≥50 kg and had clinical and/or radiological evidence of acute anterior shoulder dislocation. They were American Society of Anesthesiologists Physical Status Classification I or II and had fasted ≥90 min.2 17 The full exclusion criteria are detailed in the protocol.16
Our primary end points were patient satisfaction measured using a Visual Analogue Scale (VAS),18 and the number of patients recruited versus the number of patients screened. A member of the emergency medicine nursing team separate from the clinical or research teams asked each patient the satisfaction question. Secondary end points included the incidence and severity of adverse events as per World Society for Intravenous Anaesthesia adverse event sedation reporting tool,19 patient-reported pain score and whether the dislocation was successfully reduced or not.16 We measured overall nursing satisfaction of their experience participating in shoulder reduction using TCI propofol with a VAS. In addition, they were given the opportunity to share their views on any aspects of the procedure in free-text comments. We did not use a tool to measure clinician satisfaction instead opting for free-text comments on the data collection sheet.
Recruiting consultants were alerted to the presence of patients with a confirmed shoulder dislocation. Using the physician information sheet (online supplemental file 1), they screened the patient against the inclusion/exclusion criteria. If they were deemed eligible they would discuss recruitment to the trial with the patient and take consent if the patient were agreeable. A screening log was provided on each site to record the interaction and outcome along with reasons for refusal.
Supplemental material
When consented patient monitoring in line with current best practice2 was established. All patients received supplemental oxygen (via nasal cannulae at 4 L/min) for the duration of the sedation episode. The patient could have received morphine analgesia if it was administered at least 20 min before commencement of sedation.
Following patient enrolment, the TCI sedation flow sheet was followed (online supplemental files 2 and 3). It illustrated a step-by-step guide to the starting plasma (Cpt) target concentration of propofol, increments and upper limit Cpt. A ‘three-compartment’ pharmacokinetic model is used to allow target-controlled infusion (TCI) devices to mathematically predict the plasma concentration (Cpt) and latterly the effect site concentration (Cet), that is, the brain.15 The ‘three-compartment’ model divides the body into a central compartment (plasma), and two peripheral compartments: poorly perfused tissue, for example, body fat and highly perfused tissue, for example, brain. When a point of equilibrium is achieved, propofol will diffuse between compartments at a constant rate. These rate constants are used in the pharmacokinetic models to predict the Cpt and the Cet.
Supplemental material
Supplemental material
There are two commonly used models for propofol TCI: Schnider and Marsh. The main difference is that the Marsh model calculates the compartment volumes by the patient’s actual weight, the Schnider model takes account of other variables to calculate the compartment volumes as per the patient’s lean body mass.20 This results in a lower dose being administered. When using the Schnider model, the target is set to the effect site (Cet, brain) as opposed to the traditional plasma (Cpt) target concentration with the Marsh model. This effect-site targeting may achieve adequate sedation more rapidly.
This feasibility study used the Marsh model as we felt it wise to proceed with caution as the use of TCI in the ED is not common practice. Both versions of our TCI protocol were written with the assistance of Dr Keith Anderson, a world expert with extensive knowledge in this field. Our study of reference from which the protocol was devised involved a similarly painful procedure, oocyte retrieval, requiring procedural sedation.8
Recruiting clinicians were emergency medicine consultants. They were not involved in the reduction, their sole responsibility was the administration of TCI propofol. Training was provided for consultants that had expressed an interest in recruiting to the trial and the sessions were open to everyone in the department that wanted to learn more about TCI.
Training was delivered by an experienced anaesthetist (MABS). Training took 2 hours consisting of an interactive tutorial outlining the concept of TCI and how this would be delivered practically. Following this there was an opportunity to practice setting up the TCI pump. Three consultants were trained on each site, approximately 16% of the regional consultant workforce. Contact was maintained throughout the study with refresher sessions delivered if required.
Infusion pumps were provided by BD CareFusion to each participating department for the duration of the study. If a department were to buy the pump, the average cost would be £3000 (www.bd.com). One pump would be sufficient for the vast majority of EDs as it would be highly unusual to reduce more than one shoulder simultaneously. Bolus administration would normally require 20 mL of propofol to be drawn. Assuming that an additional 20 mL of 1% (10 mg/mL) propofol were to be drawn as standard for the TCI group, the consumables cost was estimated at £3 per patient.
Start time for the procedure was defined as the time the TCI was commenced. When the patient’s Modified Observer’s Assessment of Alertness/Sedation Scale (OAA/S)21 reached the target of three it was recorded every 3 min until the procedure was completed. Completion of the procedure was defined as the point that the TCI infusion was discontinued. A patient-reported pain score for the procedure was obtained after full recovery by nursing staff.
A formal sample size was not calculated for this feasibility study. We aimed to recruit at least 20 patients within a fixed time period to allow calculation of recruitment rate. By reviewing the average number of anterior shoulder dislocations presenting weekly at each site along with the number of recruiting consultants, the time period was agreed.
Our local data showed approximately 13 patients attending over all four sites each week with a shoulder dislocation which would equate to 845 potential patients who could have been recruited for the study period. Given the number of consultants recruiting on each site and their presence in the ED, we anticipated the number of eligible patients to be approximately 300, based on an 8-hour day shift window to reflect the typical shift duration.
Descriptive statistics were used to analyse the data.
Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
Between 3 April 2017 and 31 December 2018, we recruited 25 patients (figure 1). Six patients were recruited in the district general hospital with the urban hospitals recruiting 10, 6 and 3 patients. Recruitment was temporarily stopped between 25 April 2017 and 9 October 2017 while a drug protocol amendment was made raising the initial and maximal set plasma concentrations of propofol. Recruitment was open for approximately 16 months.
Consort flow diagram. OAA/S 3, Modified Observer’s Assessment of Alertness/Sedation Scale (OAA/S) 3; TCI, target-controlled infusion.
Screening logs were not maintained on sites. A retrospective review of audit data during the times patients could have potentially been recruited showed that there were 123 shoulder reductions undertaken implying a recruitment rate of 20%.
Two patients were excluded; one had no intravenous access and the infusion was never commenced, the other was a protocol deviation where the patient received 2% (20 mg/mL) propofol erroneously. No harm came to the patient and the incident was reported to the pharmacovigilance unit and medical ethics. The mean±SD dose of morphine administered as part of standard care at least 20 mins before commencing the protocol was 8.9±3.3 mg.
Five patients were recruited with the initial protocol (online supplemental file 1). It was noted that the maximum dose of propofol TCI (Cpt 2 µg/mL) did not enable OAA/S 3 in two out of the five patients. Recruitment was temporarily stopped and we revised the drug protocol (online supplemental file 2) raising the lower and upper limits.
Summary demographics and results for the 23 patients commenced on TCI propofol are displayed in tables 1 and 2, respectively.
Summary demographics of patients
Summary results
Twenty patients achieved an OAA/S of 3. Of the three who did not, two were prior to the protocol amendment and the third postamendment as result of the patient self-reducing when OAA/S 4. The mean±SD time to OAA/S 3 was 25±9 min. All 20 patients achieved successful reduction. The mean±SD time to reduction for the 19 recorded was 28±10 min. There were no adverse events reported.
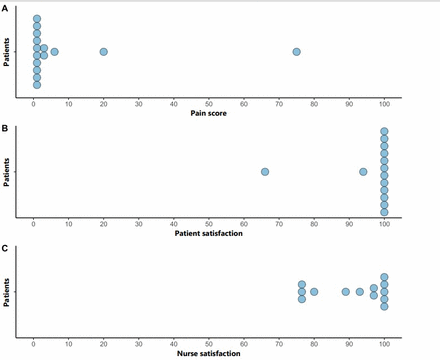
Patient-reported and nursing-reported outcomes are shown in figure 2. Overall nursing and patient satisfaction with TCI propofol and the procedure was high. Patient-reported pain scores were low, results are displayed in table 3.
Patient-reported and nursing-reported outcomes.
Patient-reported and nursing-reported outcomes
Two nurses commented that the initial stages in achieving OAA/S 3 felt slow but overall it was a better experience for the patient. Patient recall was not documented in 1 patient, present in 2 patients with a total of 17 patients reporting no recall of the procedure.
Discussion
Our small feasibility study demonstrated acceptability of the technique to the patient, successful reduction in 100% of the 20 patients achieving OAA/S 3 along with no adverse events per Society of Intravenous Anaesthesia (SIVA) adverse event reporting tool.19 These positive findings are encouraging and used in conjunction with the information we have gathered on barriers to recruitment and TCI propofol administration we can design an RCT with larger numbers.
Reported rates of adverse events during procedural sedation in the ED population ranges from 1.1% to 11%.5 6 10 22 Lower rates of adverse events have been seen in elective settings during painful procedures requiring conscious sedation such as oocyte retrieval, which has led to some interpreting the use of bolus propofol administration in ED as being high risk.7 Propofol TCI with at least one large RCT,13 in elective endoscopy procedures, showed a reduction in both respiratory and cardiovascular adverse events in comparison to the bolus administration of propofol.
Adverse events have been reported inconsistently in past studies of propofol sedation making meaningful comparisons difficult.6 Even locally, procedural sedation audits vary in their criteria between hospitals and boards. In an attempt to overcome this, the World SIVA developed an adverse event reporting tool.19 One of the participating centres uses this tool routinely and reports a minor adverse event rate of 3%, moderate adverse event rate of 0.5% and a sentinel adverse event rate of 1%. In this study, we prospectively used the SIVA adverse event reporting tool to allow standardised reporting of adverse events.
Our total recruitment was less than we expected at only 25. On review, we realised that our predicted number of eligible patients was higher than the actual number we recruited over that time period. This is partly attributable to the number of consultants recruiting and partly attributable to data collection tools. Three consultants were trained on each site, but this translated into only two on each site recruiting. There were various reasons for this with some reflecting the changing face of the consultant workforce. Our recruitment sites spanned two health boards each with different versions of electronic medical records. Each version has different mandatory input fields which meant that reports written to extract data can potentially overidentify cases based on using keywords.
While this is a small number it has afforded us valuable insight into the barriers that we need to address before embarking on our future RCT. We were unable to maintain an accurate screening log on any of the sites. Consultant presence in the clinical area did not equate to availability to actively screen. Consultant staff were challenged to screen, recruit and deliver during clinical duties. Procedural sedation on all participating sites is predominantly delivered by consultants.
Consultant availability was limited by other operational demands and they would have to weigh up departmental safety versus recruiting patients. Their primary role when on clinical duty is to maintain patient safety by supervising staff, maintaining flow and reviewing patients. A future RCT will require funding of research nurse support and research fellow time to facilitate screening, recruitment and reduce the impact of trial delivery on the patient facing clinician workforce.
Propofol TCI was not routinely used in the ED prior to this study. Enhanced training and support may have increased clinician confidence in this technique and therefore recruitment rates. As well as factoring in increased support, we will review the study design with consideration to making the RCT a stepped-wedge clustered randomised design.23 This will concentrate the training resources on each site in turn as we will implement TCI training serially rather than in parallel.
This study has several limitations. First it was designed as a feasibility study of propofol TCI and therefore it did not contain a control group. While we have shown that patients will consent to this intervention, we cannot say definitively if they would when presented with the option of being randomised between two interventions. Data are routinely gathered for all procedural sedation in the majority of UK EDs that intermittently feed into a national audit.
The measure of patient satisfaction was suboptimal as it did not take account of the complex, multidimensional nature of patient satisfaction. A future study will use the Iowa Satisfaction with Anaesthesia Scale, which is a validated tool comprising 11 items to be completed by the patient on full recovery.24
Feedback gathered from the additional comments on our data collection sheets highlighted that a major issue was the time taken to achieve OAA/S 3. While clinicians were satisfied that on reaching OAA/S 3 the procedure was smooth and the recovery quick, the time required to reach OAA/S 3 was felt to be excessive, mean±SD time 25±9 min. This compares with one participating centre’s normal practice of bolus administration being 10±6 min. To address this concern, we will review our drug protocol and consider raising the initial and maximal set plasma concentrations of propofol or if we should switch from the Marsh to the Schnider TCI model.
We plan to use these results to design a two-armed study comparing bolus propofol versus TCI propofol for sedation for reduction of anterior shoulder dislocation in the ED. The primary outcome will be incidence of adverse events with adverse events defined by the SIVA adverse event reporting tool. In order to facilitate recruitment in the challenging environment of emergency medicine, we have considered following statistical advice using a stepped-wedged cluster randomised design,25 instead of a traditional randomised controlled study design.
Considering the difficulties of maintaining a screening log and the low recruitment rates over 16 months (25 patients recruited of 123 eligible patient equating to a 20% recruitment rate) in this feasibility study, dedicated research support would be necessary for a larger RCT on multiple research sites.
Conclusion
Propofol TCI was acceptable as a method of procedural sedation for patients. Lower than expected recruitment rates highlight the need for dedicated research support.
Acknowledgments
The authors would like to thank Dr Keith J Anderson for his valuable input into the TCI drug protocols used in this study.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Handling editor Katie Walker
Twitter @al_corfield
Contributors The research idea was conceived by DJL and JM and developed by FMB and MABS. FMB drafted the manuscript with editorial input from all authors. All authors read and approved the final manuscript.
Funding Funding has been awarded by CareFusion BD.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Ethical and amendment approval was given by the West of Scotland Research Ethics Committee 5, reference number 17/WS/0020 on 24 January 2017. Individual consent from the patients was obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request. Requests for data should be made to the Chief Investigator in the first instance. Data are held in a paper format in a secure archive facility (provided by Iron Mountain, Limited) and can be retrieved within 24 working hours from this facility.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.