Article Text
Abstract
Introducion To assess the prognostic and discriminative accuracy of high-sensitivity cardiac troponin T (hs-cTnT) for prediction of inhospital mortality in emergency department (ED) patients with suspected infection.
Methods Prospective observational derivation study in ED patients with suspected infection. Prognostic performance of hs-cTnT (divided in four quartiles because of non-linearity) for prediction of inhospital mortality was assessed using multivariable logistic regression, correcting for predisposition, infection, response and organ failure (PIRO) score as a measure of illness severity and quality of ED treatment as quantified by the number of ‘Surviving Sepsis Campaign’ goals achieved. Discriminative power of hs-cTnT was assessed by receiver operator characteristics with area under the curve (AUC) analysis.
Results Hs-cTnT (median (IQR) was 57 (25–90) ng/L (n=23) in non-survivors, significantly higher than the 15 (7–28) ng/L in survivors (n=269, p<0.001). Additionally, the lowest quartile of hs-cTnT was a perfect predictor of survival because zero death occurred. Therefore, the second quartile was used as a reference category in the multivariable logistic regression analysis showing that hs-cTnT was an independent predictor of inhospital mortality: Corrected ORs were 2.2 (95% CI 0.4 to 12.1) and 5.8 (1.2 to 27.3) for the 3rd and 4th quartile compared with the 2nd hs-cTnT quartile. The AUCs of hs-TnT was 0.81 (0.74 to 0.88), similar to the AUC of 0.78 (0.68 to 0.87) of the PIRO score (p>0.05). Overall negative predictive value of hs-cTnT was 99%.
Conclusions In ED patients with suspected infection, the routinely used biomarker hs-cTnT is an independent predictor of inhospital mortality with excellent discriminative performance. Future studies should focus on the additional value of hs-cTnT to existing risk stratification tools.
Statistics from Altmetric.com
Introduction
Risk stratification of emergency department (ED) patients with a suspected infection is important for rapid initiation of adequate ED treatment and for disposition to the most optimal level of care. Erroneous disposition to a ward instead of an intensive care unit (ICU) affects patient outcome, and unnecessary admissions to a ward or ICU has a negative impact on hospital finances.1 ,2 The Mortality in ED Sepsis (MEDS), and the more recently developed Predisposition, Infection, Response and Organ-failure (PIRO) score have been developed for risk stratification of ED patients with a suspected infection.3 ,4 However, in populations meeting the criteria for early goal-directed therapy, prognostic performance might be limited.5 ,6 Moreover, both the MEDS and PIRO score were less useful for guidance of adequate disposition, while still 13% of ED patients with severe sepsis and septic shock are erroneously disposed to a normal ward, resulting in a higher mortality and longer hospital length of stay.1 Hence, the currently used MEDS and PIRO score may be strengthened by additional diagnostic tools. In a preliminary study, Kennedy et al identified seven clinical characteristics predicting an unanticipated transfer from ward to ICU. However, their model had moderate discrimination with an area under the curve (AUC) of only 0.73.7 In the present study, we therefore aimed to find a simple biomarker that could improve current risk stratification of ED patients with a suspected infection.
Impaired cardiac function has been suggested to be a crucial factor in the development of septic shock.8 ,9 During sepsis, cardiac cell wall integrity can be compromised by global hypoperfusion, and the release of endotoxins, cytokines, or reactive oxygen species,10–12 with subsequent release of troponins into the circulation. Therefore, cardiac troponin, well known for its role in risk stratification of patients with possible acute coronary syndrome, might also be a suitable biomarker for risk stratification of ED sepsis patients, with the potential to identify ED sepsis patients who need intensive care with inotropic or vasoactive medication or mechanical ventilation. Previous studies, using various types of troponin assays, showed conflicting results with regard to the prognostic performance of cardiac troponins in severely septic patients that needed ICU admission.13–17 However, none of these studies included patients with less severe sepsis stages.
The role of high-sensitivity cardiac troponin T (hs-cTnT) for risk stratification of ED patients with suspected infection has never been explored. An ED population consists of a broad severity range of the sepsis syndrome, ranging from low-risk patients who could be discharged home to septic shock patients who require ICU admission. This will affect prognostic properties of hs-cTnT. More importantly, in ED patients with septic shock, it will be clear for the ED physician that aggressive treatment and ICU admission is warranted, but especially in patients with intermediate risk, a more accurate risk stratification tool would be of great value. If hs-cTnT is an independent predictor of mortality in ED patients with a suspected infection, this routinely used biomarker could also have the potential to guide adequate disposition in less severe stages of sepsis, with the additional advantage that it is much simpler than the PIRO score.
The purpose of the present study was therefore to take the two necessary steps to investigate the potential of hs-cTnT for use in risk stratification of ED patients with a suspected infection.
First, the accuracy and discriminative performance of hs-cTnT for prediction of inhospital was assessed. Second, the association of hs-cTnT with disposition pattern and MEDS and PIRO score was assessed.
Methods
Study design and setting
The present study involves a prospective observational study in the ED to obtain a derivation cohort to assess the prognostic properties of hs-cTnt. Patients were enrolled between 1 May 2011 and 1 July 2012 at the Leiden University Medical Centre (LUMC: Tertiary care centre with ∼30 000 ED patients annually. The study was approved by the medical ethics committee of the LUMC and met the criteria for exemption from obtaining informed consent.
Selection of participants
All consecutive ED patients presenting with a suspected infection and triage categories yellow, orange and red18 were included by the triage nurse or the nurse/physician who took care of the patient. Patients with triage categories blue and green were excluded because mostly very low-risk patients were expected in this category in whom risk stratification is not likely to be an important issue (ie, patients with a simple pharyngitis). Any sign that triggered the triage nurse or treating physician to suspect an infection was suitable (ie, fever, coughing, erythema). Patients who appeared to have no infection according to the conclusion in the final hospital discharge letter were excluded (ie, pulmonary embolus, autoimmune and haematologic disorders presenting with fever).
Data collection
The nurse who took care of the patient put a patient sticker on a registration form if the patient met the inclusion criteria. Posters in the ED, and the registration form provided information on the blood that had to be obtained from the patient (including hs-cTnT, lactate and blood cultures) and the registration of vital signs and treatment variables. All nurses were informed about the data collection. However, physicians were not informed about the hs-cTnT measurement. Demographic and laboratory variables, vital signs, time to antibiotics, amount of fluid, and outcome variables were prospectively registered in the digital hospital information system (Chipsoft, Amsterdam). A medical student subsequently transferred data from the electronic hospital information system (including the electronic patient file) to a SPSS file (SPSS V.20.0, IBM, New York, USA) to calculate number of ‘Surviving Sepsis Campaign (SSC)’ goals attained19 ,20 as a measure of quality of ED treatment, and all measures of illness severity. Compliance to the SSC resuscitation bundle was quantified by assigning one point to each of the following goals attained: lactate measurement within 6 h, blood cultures before antibiotics, administration of antibiotics within 3 h, mean arterial pressure above 65 mmHg within 6 h, 1.5 L fluid bolus in case of hypotension below 90 mmHg or lactate >4 mmol/L, and ICU consultation to enable completion of the resuscitation bundle of the SSC. In The Netherlands, treatment requiring central venous and arterial catheters is usually performed in the ICU and not in the ED.
Illness severity was measured by sepsis category, similar as in the SSC,19 ,20 and by MEDS and PIRO score as described previously.3 ,4 MEDS and PIRO scores were calculated retrospectively because the scores are not used in the LUMC. The treating physician was therefore not aware of the score at the time of ED presentation. If a variable was not registered, it was counted as zero, similar as in the APACHE score.21
Hs-cTnT was measured on an autoanalyser (Cobas 8000 series, module E170) using commercial assays (Roche Diagnostics, Penzberg, Germany). The fifth-generation assay uses identical antibodies as the conventional fourth-generation one does. Minimal level of detection (lower bound) is 7 ng/L, whereas the corresponding level of conventional cTnT at the same 99% value is 10 ng/L. All Hs-cTnT levels were increased by 4 ng/L because of a previously published baseline shift of this particular assay prior to the present study.22 The decision cut-point for patients with chest pain suggestive of acute coronary syndrome is 5 ng/L. The coefficient of variation was less than 10%.
A patient was considered to have a ‘Do not resuscitate’ (DNR) status if this was already stated in the existing medical files, or when it was decided at the time of ED presentation or during hospital admission. Disposition was defined as the initial destination of the individual patient after ED presentation. The final hospital discharge diagnosis was derived from the hospital discharge letter.
Outcome measures
The primary outcome measure was in-hospital mortality.
The secondary outcome measure was disposition pattern of ED patients with a suspected infection, because if hs-cTnT is to be used to help in adequate disposition, hs-cTnT should also correlate with disposition to home, hospital ward or ICU.
It was investigated whether unanticipated transfers from ward to ICU occurred, and to assess if there was indeed a patient group that survived on the ICU without any specific ICU-related intervention. ED patients who were initially admitted to the ward but had an unanticipated transfer from the ward to the ICU, or who died in the ward (while not having a DNR status) were considered as patients who should have been admitted to the ICU. Patients who were admitted to the ICU for less than 24 h without receiving mechanical ventilation, vasoactive or inotropic medication, or venovenous haemodialysis, were considered as patients who could have been safely admitted to the ward (‘unnecessary’ ICU admissions). Although observation is a very important function of the ICU, it is likely that these patients would have survived in a hospital ward. Patients were followed-up until hospital discharge.
It was left at the discretion of the treating physician to consult the ICU. Hs-cTnT is not used for risk stratification in our hospital. After hospital discharge, it was checked if a patient had an unanticipated transfer to the ICU during the admission on the ward due to deterioration of their sepsis.
Data analysis
Data were presented as mean (SD) if normally distributed and median (IQR) if data were rightly skewed. Descriptive categorical data were analysed using χ2 tests. Continuous data were analysed with a Student t test, or Mann–Whitney test, as appropriate. The Kruskal–Wallis ANOVA was used for comparisons of hs-cTnT levels among the four groups of PIRO, MEDS, sepsis and disposition category, because the distribution of hs-cTnT was rightly skewed. Multivariable binary logistic regression was used to assess if hs-cTnT was an independent predictor of inhospital mortality.
A prediction model was constructed using forward entry of variables with p<0.2 in the univariate analysis. Because we wanted to explicitly compare hs-cTnT with the PIRO score, PIRO score was put in the model as a summary variable of illness severity instead of all separate components of PIRO. Because the presence of myocardial and renal disease could be a potential confounder of the association between hs-cTnT and inhospital mortality, both predisposition variables were put in the model besides the PIRO score. DNR status was expected to be colinear with PIRO score, and was therefore not put in the model. Quality of ED treatment, as quantified by the number of SSC goals attained was also put in the model because this was expected to affect outcome. All independent variables were first ranked into four quartiles to check for linearity, before they were entered in the model. Because hs-cTnT was not linear, the dummy variables corresponding with the four quartiles were used for the final model. Because the first quartile of hs-cTnT appeared to be a perfect predictor of survival (no patients died in the lowest quartile of hs-cTnT), only the dummy variables of the 2nd, 3rd and 4th quartiles were put in the final model with the 2nd quartile being the reference category. The Hosmer–Lemeshow test was used as an overall measure of model calibration.
Sample size was calculated using the general rule of thumb of number of events divided by 10. Because we wanted to correct for initial illness severity and quality of ED treatment, approximately 20–30 events were needed.
ORs (95% CI) were reported for the crude and corrected models. p<0.05 was considered to be significant.
Discriminative performance of hs-cTnT was assessed by using receiver operator characteristics (ROC) with AUC analysis with inhospital mortality as outcome.
All data were analysed using SPSS statistics V.20.0.0 software (IBM, New York, USA).
Results
Patient characteristics and inclusion
A total of 404 patients were included. In 292 patients, hs-cTnT was measured. Mean age was 57 (18) years, with 214 (53%) males.
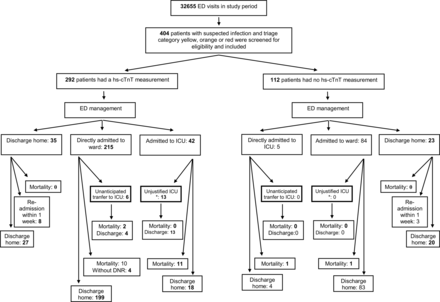
Figure 1 shows patient inclusion and outcome as a function of disposition; 7.4% (16 of 215) of the ED patients with a suspected infection had an unanticipated transfer from ward to ICU or died on the ward, of whom 10 of 16 did not have a DNR status. Conversely, ∼32% (13 of 41) of ED patients with a suspected infection were admitted to the ICU shorter than 24 h, and without specific ICU-related interventions. In web appendix 1, patient characteristics of included patients with and without hs-cTnT measurement are summarised. Table 1 shows patient characteristics of included patients.
Patient characteristics
Patient inclusion and flow through the study. *Surviving patients who were admitted to the ICU for less than 24 h, and who were merely observed in the ICU. Patients with no hs-cTnT measurement are shown in web appendix 1. ICU, intensive care unit; ED, emergency department.
The association of hs-cTnT with illness severity and disposition
First, it was tested whether hs-cTnT was associated with the level of illness severity and disposition, because this is one minimal prerequisite if hs-cTnT is to be used as a tool to guide adequate disposition. Hs-cTnT was significantly associated with validated measures of illness severity, that is, sepsis category, MEDS and PIRO score (figure 2A–C; p<0.001). Additionally, figure 2D shows that hs-cTnT levels increased with disposition category (p<0.001).
Hs-cTnT levels as a function of illness severity. Illness severity was quantified by PIRO (A) and MEDS (B) score, and sepsis (C) and disposition (D) category. The whisker-boxplots show median (IQR and 95% CI). Circles and stars represent outliers. Kruskal–Wallis ANOVA to test differences among groups. MEDS, mortality in emergency department sepsis score; PIRO, Predisposition, Infection, Response, Organ-failure score; ICU, intensive care unit.
Accuracy and discriminative power of hs-cTnT for prediction of inhospital mortality
Figure 3 summarises the prognostic performance of hs-cTnT. In figure 3A, it is shown that hs-cTnT levels are significantly higher in non-survivors compared with survivors. Discrimination of hs-cTnT is excellent, and comparable to the discriminative performance of the more complex PIRO and MEDS scores (figure 3B, p>0.05). Finally, figure 3C reveals that hs-cTnT is an independent predictor of inhospital mortality, when corrected for illness severity (PIRO score) and ED treatment (number of SSC goals attained). The lowest quartile of hs-cTnT (<7 ng/L) was a perfect predictor of survival.
Prognostic performance of hs-cTnT. (A) Hs-cTnT levels of survivors versus non-survivors. Data are presented as median (95% CI). Circles and stars represent outliers. (B) Receiver operator characteristics (ROC) of hs-cTnT, Predisposition, Infection, Response and Organ-failure (PIRO) score and Mortality in Emergency Department Sepsis (MEDS) score for prediction of inhospital mortality as outcome. Do not resuscitate patients were excluded from analysis. Data are presented as mean (95% CI) area under the curve (AUC). (C) Univariate (crude model) and multivariate (corrected model) logistic regression. Prediction model corrected for illness severity (PIRO) and quality of emergency department treatment (Surviving Sepsis Campaign (SSC) goals attained). Hosmer and Lemeshow tests for crude and corrected models were p=1.0 and p=0.19, respectively.
The hs-cTnT level at maximal sensitivity and specificity in the ROC plot (thus the upper left corner) was 18 ng/L. The negative predictive value at this cut-off value was 0.99 (0.98–1.0).
Limitations
There are a several limitations to our study. First, in ∼28% of the patients eligible for inclusion, hs-cTnT was not measured despite the prospective nature of the database and the instructions to nurses to measure hs-cTnT. The patients in whom hs-cTnT was measured had similar patient characteristics as patients in whom hs-cTnT was not measured, except for the presence of pneumonia, respiratory difficulty and mortality (see web appendix 1). It is unlikely, however, that selection bias has occurred in the present study for several reasons:
First, selection bias would only have occurred if patients with low illness severity and a high hs-cTnT, or patients with high illness severity and a low hs-cTnT would have been selectively excluded. The chance that this occurred in our study is extremely small, especially because nurses and doctors were not informed about the reason for hs-cTnT measurement. Second, imputation of the median hs-cTnT for the 28% patients without a hs-cTnT measurements, does hardly affect the prognostic performance of hs-cTnT, while this is a worst-case scenario, because in the majority of patients with low illness severity, the median hs-cTnT is likely to be too high, and for the minority of cases with high illness severity the median is much too low (see web appendix 2).
Discussion
The main finding of the present study is that the routinely used cardiac biomarker hs-cTnT is an independent predictor of inhospital mortality in ED patients with a suspected infection.
The necessity of improving risk stratification of ED sepsis patients
Approximately 5% (10 out of 215, see figure 1) of the ED patients with a suspected infection had an unanticipated transfer from ward to ICU or died on the ward while not having a DNR status, similar as the number that was found by Kennedy et al.7 The observation that ∼33% (2 out of 6) of the ED patients with an unanticipated transfer died in the ICU, versus ∼26% of the patients directly admitted to the ICU corresponds to previous studies,1 ,2 and underlines the importance of improving risk stratification of ED sepsis patients.
Conversely, ∼32% (13 out of 42, see figure 1) of ED patients with a suspected infection were admitted to the ICU shorter than 24 h, and without specific ICU-related interventions. Although observation is an important function of ICUs, it is likely that these patients would have survived on a normal ward, at much lower costs, which is important because the healthcare-associated costs for sepsis are still increasing.23 Thus, there is still a need for improvement of risk stratification of ED sepsis patients.
The prognostic performance of hs-cTnT in ED sepsis patients
The present study is the first to show that hs-cTnT is an independent predictor of inhospital mortality in ED patients with a suspected infection, with similar accuracy and discriminative power as the MEDS and PIRO scores (figure 3).3 ,4 ED patients with an hs-cTnT in the lowest first quartile (<7 ng/L) could have been safely discharged home directly from the ED with a 100% specificity for survival. ED patients with a hs-cTnT in the fourth quartile (70 ng/L) have a 5.8 times higher odds to die in the hospital compared to patients with a hs-cTnT in the second quartile (11 ng/L), even when initial illness severity and ED treatment is taken into account.
Several studies found troponin to be an independent predictor of mortality in ICU patients with severe sepsis or septic shock, consistent with the present study.13–15 However, our findings are in contrast with the study of Rosjo et al and Triuvoipati,16 ,17 who found that hs-cTnT was not an independent predictor of mortality in ICU patients with septic shock. This discrepancy is probably caused by the differences in patient populations and the different troponin assay used. Our ED population contained a much wider severity range of septic patients, including low-risk patients. Patients with septic shock will be easily recognised by the ED physician, and additional risk stratification tools are unlikely to contribute to optimising adequate disposition. Especially in the intermediate risk groups, additional aids in decision making are likely to contribute to optimise initial treatment and safe and cost-effective disposition.
Additional value of hs-cTnT to risk stratification by PIRO score
Howell et al4 proved that PIRO is a valuable concept for risk stratification of ED patients with a suspected infection, but also suggested that it might be fine-tuned by adding biomarkers, or other variables. In the present study, the first necessary step was undertaken to investigate the potential of hs-cTnt for use in risk stratification of ED patients with a suspected infection. Although hs-cTnT is much simpler than the PIRO score and appears to have similar accuracy and discriminative performance, we do not advocate replacement of the PIRO score because PIRO should be used as a classification, with each component giving different information that can be used by the ED physician for decisions for the individual patient. We rather suggest that hs-cTnT could be added to the PIRO score, that is, the organ failure component, if future studies confirm our findings.
In conclusion, the routinely used cardiac biomarker hs-cTnT is associated with illness severity and disposition, and represents an independent predictor of inhospital mortality in ED patients with suspected infection, with similar prognostic performance as the PIRO and MEDS scores. Future studies should confirm whether incorporation of hs-cTnT in the PIRO score increases the prognostic power of the PIRO classification, or whether hs-cTnT might be used as an independent risk stratification tool.
Acknowledgments
We are grateful to all the nurses, staff members, senior house officers and residents who were involved in patient inclusion.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online supplement
Footnotes
-
BDG and RCW contributed equally to this study.
-
Contributors BDG invented the study idea, designed the study, collected data, contributed to the analysis and edited the manuscript. RCWV collected data, did the analyses and wrote the manuscript. JL collected data. JVDV contributed to the study idea and edited the manuscript. BDG takes full responsibility for the study as a whole.
-
Competing interests None.
-
Ethics approval Medical ethical committee of the Leiden University Medical centre.
-
Provenance and peer review Not commissioned; externally peer reviewed.