Article Text
Statistics from Altmetric.com
- high sensitivity troponin T
- differential diagnosis
- false positive troponin
- analytical interference
- systemic sclerosis
Cardiac troponin (cTn) is a regulatory protein of the myofibrillar thin filament of striated muscle regulating excitation–contraction coupling in the heart.w1 Among the three subunits (T, I, and C), only cardiac troponin T (cTnT) and I (cTnI) are expressed in cardiac muscle and released into blood following myocardial cell death. Several distinct pathobiological mechanisms leading to elevated troponin values have been suggested, not all of which involve myocyte necrosis.w2
cTnT or cTnI are routinely used in emergency units as the preferred biomarkers for the diagnosis of acute myocardial infarction (MI). According to joint criteria for the diagnosis of acute MI by the European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force, an acute MI should be diagnosed in patients with symptoms of myocardial ischaemia and detection of a rise and/or fall of cardiac biomarkers (preferentially troponins) with at least one value above the 99th percentile of the upper reference limit.1
Use of high sensitivity troponin assays allows more accurate and earlier detection of MI.2 w3 Higher analytical sensitivity increases the number of patients with analytically true positive cTn results due to non-ST elevation MI (NSTEMI) but also due to numerous acute or chronic diseases in the absence of overt ischaemic heart disease (box 1).3 w4
Elevations of cardiac troponin values because of myocardial injury
Injury related to primary myocardial ischaemia
Plaque rupture
Intraluminal coronary artery thrombus formation
Injury related to supply/demand imbalance of myocardial ischaemia
Tachy-/bradyarrhythmias
Aortic dissection or severe aortic valve disease
Hypertrophic cardiomyopathy
Cardiogenic, hypovolaemic, or septic shock
Severe respiratory failure
Severe anaemia
Hypertension with or without left ventricular hypertrophy
Coronary spasm
Coronary embolism or vasculitis
Coronary endothelial dysfunction without significant coronary artery disease
Injury not related to myocardial ischaemia
Cardiac contusion, surgery, ablation, pacing, or defibrillator shocks
Rhabdomyolysis with cardiac involvement
Myocarditis
Cardiotoxic agents, for example, anthracyclines, trastuzumab (Herceptin)
Multifactorial or indeterminate myocardial injury
Heart failure
Stress (takotsubo) cardiomyopathy
Severe pulmonary embolism or pulmonary hypertension
Sepsis and critically ill patients
Renal failure
Severe acute neurological diseases, for example, stroke, subarachnoid haemorrhage
Infiltrative diseases, for example, amyloidosis, sarcoidosis
Strenuous exercise
Electroconvulsive therapy
Modified from Thygesen et al1 with permission of Oxford University Press.
The cause of cTn elevations may not always be clearly identifiable. Even in the general population elevated values of high sensitivity troponin T have been reported, which were associated with an increased risk for all cause mortality and/or structural heart disease.w5 w6 In an elderly community population of 70-year-old subjects elevated cTnI concentrations were reported in 22%.w7 Although they were more frequently observed in the presence of cardiovascular risk factors, impaired left ventricular (LV) systolic function, increased LV mass, and other comorbidities in this study, the cause of frequently elevated cTn in the elderly is still under debate. It remains unresolved as to whether this can be fully attributed to an age related increase of comorbidities, or whether it is at least partly due to the physiologic aging processes. Either way, we recommend proper further diagnostic workup in this high risk subpopulation. Importantly, diagnostic accuracy of sensitive cTn assays is still high for MI in the elderly. However, a receiver operating characteristic (ROC) curve optimised cut off for the diagnosis of NSTEMI in patients >75 years was reported to be twofold higher than the 99th percentile.4 ,5
In cases where troponin results and clinical presentation are strikingly discordant, analytically false positive or negative cTn values have to be considered. As truly elevated cTn is associated with an adverse outcome regardless of the underlying condition, the exact reason for cTn elevation has to be investigated.w8−w11
Recommendations for further laboratory evaluation of an analytically false positive cTn value are limited. This article aims to provide an overview of the potential reasons for preanalytical and analytical errors, summarise methods, and suggest an algorithm for the detection of false positive troponin measurements. To illustrate the problem of differentiating false positive from true positive troponin results, we present a clinical case.
Clinical case
A 53-year-old Caucasian female was admitted to hospital after detection of elevated high sensitivity cardiac troponin T (hsTnT) values up to 1.874 pg/ml (99th percentile: 14 pg/ml) without a previous history of cardiac disease. The patient reported dyspnoea during mild exercise and weight loss of 16 kg (19% of initial bodyweight) within the past 6 months without elevated temperature or night sweats. No other complaints were reported. The patient had initially been hospitalised for exclusion of suspected pulmonary fibrosis in the pulmonary department. During diagnostic workup, N-terminal pro B-type natriuretic peptide (NT-proBNP) and creatinine kinase had been significantly elevated which had prompted the measurement of hsTnT.
Clinical examination was unremarkable except for a soft systolic murmur heard loudest over the right second intercostal space; there was no cyanosis, no peripheral oedema, and the lung bases were clear.
Echocardiographic findings showed normal LV and right ventricular (RV) function with an LV ejection fraction of 65%. On coronary angiography there was no significant coronary artery disease. For further evaluation cardiac MRI (cMRI) using a 1.5 T whole body scanner was performed. The morphological scans showed no significant pericardial effusion and in the T2 weighted images there were no signs of myocardial oedema. Late gadolinium enhancement (LGE) imaging was performed, but due to impaired image quality a pericarditis could not be excluded definitively.
Myocardial biopsy specimens were obtained 11 days after the initial cMRI to diagnose myocarditis. RV myocardial biopsies were obtained according to the discretion of the interventionist, as there are currently no clear recommendations for LV versus RV biopsy.w12 Abdominal ultrasound demonstrated a mild hepatosplenomegaly. On spiroergometry a restrictive ventilatory defect was detected that was in keeping with suspected pulmonary fibrosis.
Due to the discrepancy between clinical presentation, normal findings in coronary angiography, cMRI and echocardiography, and very high hsTnT concentrations, measurement of hsTnT was repeated, confirming very high values. Additionally, a cTnI test was performed from the same sample using the Siemens cTnI Ultra Assay, showing only a minimal elevation at 50 pg/ml (99th percentile: 40 pg/ml). Due to the oligosymptomatic clinical presentation and the equivocal elevation of hsTnT and cTnI, an analytically false positive troponin T result was suspected.
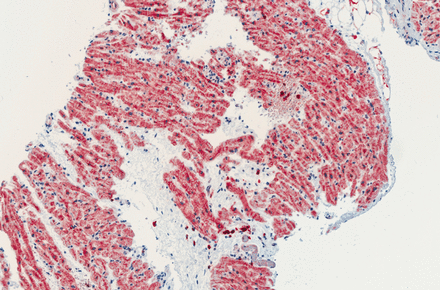
However, more extensive laboratory tests revealed high titre antinuclear antibodies (1 : 20 000) and mild elevations of further markers of autoimmune disease. cMRI with LGE imaging was repeated on the day the biopsy specimens were taken and showed an enhancement of the lateral and inferolateral basal and midventricular pericardium corresponding to an acute pericarditis (figure 1). Biopsy results became available proving cardiac pathology with signs of inflammatory cardiomyopathy showing three infiltrates with >14 CD3 and CD68 cells in all biopsies together. According to the World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies, myocarditis is present if focal or diffuse mononuclear infiltrates with >14 leucocytes/1 mm2 (CD3 T lymphocytes and/or CD68 macrophages) are detected with enhanced expression of HLA (human leucocyte antigen) class II molecules (figure 2).6 w13 w14
Four chamber view acquired by a 1.5 T whole body MRI showing late enhancement and thickening of the lateral pericardium.
Immunohistochemical analysis of biopsy specimen from the right ventricular endomyocardium: augmented interstitial CD3 T cells marked by dark red/brownish colour. Twenty-one CD3 T lymphocytes/mm2 were counted in total, fulfilling the criteria for inflammatory cardiomyopathy. The skilful technical assistance of John Moyers is gratefully acknowledged.
The results of the second cMRI were compatible with the biopsy results; due to the diffuse myocardial involvement in perimyocarditis the extent of LGE does not necessarily correlate with the degree of cellular inflammation and necrosis.w15
Troponin assays from serum and plasma aliquots were measured at two different time points (table 1). Also listed is the value for C reactive protein, indicating a low level of inflammation.
Test results
A substantially elevated troponin I value was detected using the Mitsubishi PATHFAST assay with a result of 742 pg/ml (99th percentile: 29 pg/ml, 26 × ULN (upper limit of normal)). Using the Architect prototype hsTnI assay (Architect STAT High Sensitive Troponin, Abbott Diagnostics), a value of 13.7 pg/ml was measured (limit of detection: 3.4 pg/ml; for this test the 99th percentile was determined to be 30 pg/ml in a reference population of 4139 individuals in the population based Gutenberg Health Study).w16
A follow-up blood sample was drawn after 8 months, which continued to show an elevation of hsTnT (377 pg/ml), while the troponin I assays showed negative results (TnI Ultra, Siemens: <40 pg/ml; Architect STAT High Sensitive Troponin: 5.1 pg/ml).
Thus, the troponin I assays ranged from below the ULN (Architect STAT High Sensitive Troponin, Abbott Diagnostics) to 26 × ULN (Mitsubishi Pathfast), compared to an elevation of hsTnT up to 162 × ULN.
Taking into consideration all available laboratory, clinical and imaging information, a final diagnosis of systemic sclerosis with pulmonary and cardiac involvement was made. After initiation of corticosteroid therapy, troponin values decreased and dyspnoea resolved, while progressive skin sclerosis became evident. The patient continues to be treated in the rheumatologic outpatient clinic with immunosuppressive therapy.
Approach to the patient with a suspected false positive troponin result
Troponin values that are strikingly discrepant with the clinical picture should raise the suspicion of an analytical error. A meaningful laboratory approach to exclude analytical errors involves initially ruling out simple causes. The measurement should be repeated to rule out a ‘wrong blood in tube’ (WBIT)-type error, where the blood sample does not contain the blood of the patient identified on the tube.
As highly sensitive troponins are able to detect very early stages of disease, the use of the most sensitive and specific diagnostic modalities may be necessary when testing for causes of troponin elevations. When echocardiography is inconclusive, contrast enhanced high resolution MRI may be indicated, as this technology is the reference method for RV and LV morphology and function, and allows unique tissue characterisation using contrast enhancement.7 w17 w18 Assomull et al8 found an identifiable basis of troponin elevation through cMRI in 65% of 60 consecutive patients with elevated troponin concentrations without significant obstructions on coronary angiogram.w19
Endomyocardial biopsy has the strongest indication for surveillance of cardiac allograft rejection.w20 The American Heart Association, the American College of Cardiology, and the European Society of Cardiology (ESC) launched a recommendation for endomyocardial biopsy with a class I indication for patients with new onset heart failure of <2 weeks’ duration associated with a normal sized or dilated left ventricle and haemodynamic compromise; or for patients with new-onset heart failure of 2 weeks’ to 3 months’ duration associated with a dilated left ventricle and new ventricular arrhythmias, second or third degree heart block, or failure to respond to usual care within 1–2 weeks.9 Although classes of recommendation are lower for other clinical scenarios, endomyocardial biopsy may also provide valuable information in carefully selected patients using routine light microscopic examination with staining for detection of myocarditis or amyloidosis, and using histochemistry and immunohistochemistry for suspected myocarditis, storage diseases, tumour typing, amyloid classification, or viral genome analysis.w21 w22
Kinetic changes of troponin concentrations in serial blood sampling will often aid in differentiating acute from chronic cardiac disease, but also AMI from non-AMI related troponin elevations.10 w23 In the absence of a clinical history, ECG evidence of ischaemia, structural heart disease, renal failure or other plausibile explanations, analytically false positive results should be considered.
Analytical issues
This section discusses analytical issues potentially causing interference in troponin assays. After a general overview some specific factors are discussed in more detail with a focus on interfering antibodies, questionable cross reactivities in skeletal muscular disease, and differences between troponin assays.
General overview
Several causes of false positive cTnI or cTnT have previously been summarised by Lum et al11 (box 2).
Possible causes of false positive troponin results
-
Heterophile antibodies
-
Human anti-mouse antibodies
-
Autoantibodies
-
Rheumatoid factor
-
Haemolysis
-
Fibrin clots
-
Microparticles in specimen
-
Interference by endogenous components in blood (bilirubin, haemoglobin, lipaemia)
-
High concentration of alkaline phosphatase
-
Immunocomplex formation
-
Analyser malfunction
Modified from Lum et al11 with permission of the American Society for Clinical Pathology.
Haemolysis should be considered as a potential cause of lower or higher concentrations of cTn and may occur due to incorrect blood draw, transport or storage. The rate of haemolysis has been reported to be higher in emergency departments compared to wards or outpatient phlebotomy services.w24 Systematic identification of unsuitable specimens by standardised use of tools such as the haemolysis index have been recommended.w25 The confounding influence of haemolysis on interpretation of serial changes in troponins may depend on which assay is being used.w26 Tube systems used for transportation have been suspected as relevant potential causes of haemolysis.w27 w28
The choice of blood collection tubes may also be a cause of interference, especially in earlier troponin assay generations.w29 With the third generation Elecsys Troponin T immunoassay, heparin plasma collection tubes were associated with systematic lower test results because of a direct interference of the immunoassay by heparin. This problem was solved in the fourth generation Elecsys Troponin T assay, which showed comparable cTnT results determined in heparin plasma and serum.w30
Excess fibrin has also been described as a cause of false positive cTnI results due to non-specific antibody binding, or to trapped indicator enzyme in the separation matrix in incompletely clotted serum samples.w31
Positive interference may also occur due to high concentrations of alkaline phosphatase in some cTnI assays using alkaline phosphatase as a substrate, or to the presence of a macro immunocomplex involving cTnI and IgG.w32 w33
Interfering antibodies
Heterophilic antibodies, presumably one of the most frequent causes of false positive results, are endogenous human antibodies that have the ability to bind to immunoglobulins of other species. Binding of heterophilic antibodies to antibodies used as reagents in immunoassays may lead to positive test results independent of the concentration of the true analyte.12 Some heterophilic antibodies are polyspecific with only moderate affinity to animal antigens or autoantigens. While their affinity is usually too low to cause interference, their concentrations may significantly rise in certain settings such as viral infections. In contrast, human anti-animal antibodies (HAAA) are highly specific with high affinity against antigens from specific animal species, often causing significant interference, most commonly as human anti-mouse antibodies (HAMA). These may be present due to previous therapy with monoclonal antibodies, blood transfusions, vaccinations against infectious diseases, maternal transfer across the placenta to the unborn child, exposure to microbial antigens, keeping of pets, or autoimmune diseases associated with elevated autoantibodies (eg, rheumatoid factor).13 ,14 HAMA may cause both false positive and false negative interference in assays based on two-site mouse monoclonal antibodies.w34
Most immunoassays for cTn are based on such a double ‘sandwich’ method, where two distinct antibodies bind to different epitopes of the same analyte. While an immobilised first antibody captures any troponin present, the second labelled antibody serves as a detection molecule, generating a measurable signal after a last wash phase.11 In the presence of heterophilic and HAAA, crosslinks to either one of these antibodies may lead to a false positive signal in the absence of the target analyte.w35 Although many manufacturers add blocking agents to their assays to limit interferences, a false positive signal may be generated when large quantities of heterophilic antibodies are present. Lum et al11 suggest possible approaches to test and account for these interferences, such as using another manufacturer's assay, using blocking agents, or adding endogenous immunoglobulin-free serum samples.w36 In the Cobas Troponin T high sensitivity assay a mouse–human chimeric detection antibody is used to eliminate further potential interference by heterophilic antibodies.w37
The incidence of heterophilic antibodies is not known precisely and varies in studies, ranging from 0.17–40%.w38 w39 In populations of apparently healthy subjects, Adamczyk et al14 reported a similar prevalence of autoantibodies reactive with cTnI (12.7%) and autoantibodies reactive to cTnT (9.9%) in customised, direct chemiluminescent microplate assays carried out on a Berthold Mithras microplate reader. In these tests a murine antihuman IgG (subtype IgG2b, kappa, Abbott Diagnostics Division) was used as the detection conjugate, and recombinant human cTnT and cTnI (BiosPacific, Emeryville, California, USA) were used as capture antigens.w40
Questionable cross reactivities in skeletal muscular disease
A potential interference of particular interest is suspected crossreactivity with cTnT in skeletal muscular disease. The specificity of troponins is a major advantage compared to other markers of cardiac injury, such as creatine kinase and lactate dehydrogenase.w41 w42 Recent reports by Jaffe et al15 and Sribhen et alw43 have suggested that elevations of serum cTnT may be caused by re-expression of cTnT in diseased skeletal muscle leading to a false positive diagnosis of cardiac injury. In our opinion this hypothesis is interesting but not supported by the results presented due to crucial methodological limitations, as we have previously reported.16 Observed elevations of cTnT in some patients with skeletal muscle myopathy or dystrophy can most likely be explained by myocardial involvement due to a systemic disorder. The possibility of re-expression of cTnT in skeletal muscle nevertheless merits further investigation.
Analytical differences between troponin assays
Multiple commercial assays by different manufacturers are in use for cTnI while cTnT assays are made only by a single manufacturer. Available troponin assays on the market have different sensitivities and do not all meet the minimum analytical criteria suggested in current guidelines.
The term ‘high sensitivity’ should only be used for assays with a total imprecision at the 99th percentile ≤10% and measurable normal values below the 99th percentile in at least 50% of healthy individuals.17 A recent publication on behalf of the IFCC Task Force on Clinical Applications of Cardiac Biomarkers lists the currently marketed troponin assays, as well as the newer high sensitivity assays available.18
While the overall prevalence of false positive cTnT and cTnI is unknown, false positive troponin I elevations of up to 3.1% have been reported in reference populations.w44 w45 Antibodies with varying epitope specificities used in different cTnI assays have been suggested as an important cause of discrepant test results. Post-translational modifications such as proteolytic degradation and phosphorylation of cTnI may lead to changed cTnI epitopes.w46 w47 Such modifications are less likely to affect test results if the antibodies that are used are specific to epitopes located in the central part of troponin molecules. Attempts of one manufacturer testing hundreds of combinations of two-site monoclonal antibodies (MAb)—with the goal of finding a single combination of a capture and a detection antibody completely insensitive to any cTnI modifications—have not been successful, leading to a recommendation to develop cTnI assays with two different MAbs for capture and two MAbs for detection to minimise interference.w48
Case discussion
Although the high sensitivity troponin T assay used is considered more sensitive than the troponin I assays utilised, the striking discrepancy between elevated hsTnT and disproportionally low cTnI cannot be explained by the varying assay sensitivities alone. As biopsy specimens showed clear signs of cardiac pathology, it is highly likely that the only borderline elevations of troponin I assays employed are due to test-specific interferences, although we cannot determine the exact mechanism. Additional contributing factors are still elusive and merit further investigations.
Suggestions for clinical practice
Highly sensitive troponin assays have a higher sensitivity for detection of myocardial injury, be it due to acute coronary syndrome (ACS) or non-ACS conditions, and may also improve prognostic assessment.19 While it is not feasible to recommend the preferential use of troponin T or troponin I, the use of cTn assays approved for routine diagnostics, as listed in a recent paper published by the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care, should be advocated.20
Increased sensitivity allows earlier and more frequent detection of NSTEMI but also detection of hitherto undetected cardiac pathologies in the absence of ischaemic cardiac disease. This increase of sensitivity is a challenge for most contemporary diagnostic methods and prompts the need for more sensitive and specific imaging technologies including cMRI and coronary CT angiography.w49 As these technologies may not be readily available in most hospitals, a mislabelling of ‘true positives’ as ‘analytically false positives’ may occur. We suggest the use of a simple algorithm whenever laboratory findings are incompatible with clinical findings and an analytical error is suspected (box 3)
Algorithm for assumed false positive troponin result due to discrepancy between clinical presentation and laboratory value
Suspected false positive troponin result
↓
Same test result in repeated blood draw? Haemolysis?
↓
Rule out true cardiac pathology (1)
Check for non-acute coronary syndrome related causes of elevated troponin (echocardiography, further laboratory testing, etc)
↓
Consider analytically false positive result
Test sample with different troponin assay (if available)
Directly communicate with laboratory to: (1) consider adding endogenous immunoglobulin-free serum samples or using blocking agents; (2) check analyser to rule out instrument malfunction; (3) examine specimen tube for microparticles or clots to rule out fibrin interference (centrifuge again if necessary); (4) refer further testing to reference laboratory or manufacturer
↓
Rule out true cardiac pathology (2)
Consider further cardiac testing using more sensitive methods (cardiac MRI, coronary CT angiography, coronary angiography, myocardial biopsy)
Direct communication between clinicians and laboratory departments will allow timely interdisciplinary assessment and help prevent unnecessary and potentially harmful diagnostic and therapeutic procedures.
False or analytically true positive elevations of cardiac troponins: key points
-
Consider analytically false positive cardiac troponin (cTn) values when troponin results and clinical presentation are strikingly discordant.
-
Since truly elevated cTn values of any cause are associated with an adverse outcome, other differential diagnoses must be actively sought.
-
Interdisciplinary assessment of clinicians and their laboratory departments may prevent unnecessary and potentially harmful diagnostic and therapeutic procedures.
You can get CPD/CME credits for Education in Heart
Education in Heart articles are accredited by both the UK Royal College of Physicians (London) and the European Board for Accreditation in Cardiology—you need to answer the accompanying multiple choice questions (MCQs). To access the questions, click on BMJ Learning: Take this module on BMJ Learning from the content box at the top right and bottom left of the online article. For more information please go to: http://heart.bmj.com/misc/education.dtl
▸ RCP credits: Log your activity in your CPD diary online (http://www.rcplondon.ac.uk/members/CPDdiary/index.asp)—pass mark is 80%.
▸ EBAC credits: Print out and retain the BMJ Learning certificate once you have completed the MCQs—pass mark is 60%. EBAC/ EACCME Credits can now be converted to AMA PRA Category 1 CME Credits and are recognised by all National Accreditation Authorities in Europe (http://www.ebac-cme.org/newsite/?hit=men02).
Please note: The MCQs are hosted on BMJ Learning—the best available learning website for medical professionals from the BMJ Group. If prompted, subscribers must sign into Heart with their journal's username and password. All users must also complete a one-time registration on BMJ Learning and subsequently log in (with a BMJ Learning username and password) on every visit.
Acknowledgments
We thank Dr Klaus Hallermayer from Roche Diagnostics GmbH, Werk Penzberg, Germany, Dr Tanja Zeller from University Heart Center Hamburg and Dr Eberhard Spanuth for their expert advice and performing various tests on our patient's blood samples.
References
- ↵Updated global approach to diagnosis of myocardial infarction by the joint European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force.
- ↵
- ↵A well structured expert consensus document about the proper use of cardiac troponins in the setting of acute cardiac care.
- ↵
- ↵
- ↵
- ↵Comprehensive clinical competence statement on Cardiac Imaging with CT and MRI of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training.
- ↵
- ↵
- ↵
- ↵Important paper on false positive cardiac troponin results in patients without myocardial infarction with overview of possible interferences.
- ↵A detailed and comprehensive overview on heterophilic antibodies suggesting that infrequent heterophilic interference cannot be avoided.
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
- ↵
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Files in this Data Supplement:
- Data supplement 1 - Online references
Footnotes
-
Contributors MV and EG were involved in the conception, design and drafting of this manuscript. MV, MB, MM, PAS, FA, HS, MZ, MS, SB, HAK and EG critically revised the draft manuscript. FA and HS prepared and interpreted the sections on MRI. All the authors read and approved the final manuscript.
-
Competing interests In compliance with EBAC/EACCME guidelines, all authors participating in Education in Heart have disclosed potential conflicts of interest that might cause a bias in the article. EG is a consultant to Roche Diagnostics and Brahms Biomarkers and has received speakers’ honoraria from Roche Diagnostics, Siemens Healthcare, Brahms Biomarkers, and Mitsubishi Chemicals. HAK has developed the cardiac troponin T assay and holds a patent jointly with Roche Diagnostics. He has received grants and research support from several companies, and has received honoraria for lectures from Roche Diagnostics. SB reported receiving lecture fees from and consulting for Brahms Thermo Fisher and receiving lecture fees from Abbott Diagnostics and Siemens. MV has been reimbursed for travel expenses and fees associated with attending seminars and conferences by Octapharma, Lilly Germany, GlaxoSmithKline, Roche Diagnostics, Brahms, Leo Pharma, and Abbott. All other authors declared no competing interests.
-
Provenance and peer review Commissioned; externally peer reviewed.